当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
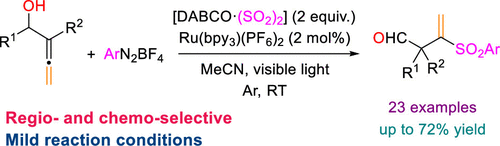
Visible-Light-Mediated Ru-Catalyzed Synthesis of 3-(Arylsulfonyl)but-3-enals via Coupling of α-Allenols with Diazonium Salts and Sulfur Dioxide
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.orglett.0c03482 Fernando Herrera 1 , Amparo Luna 1 , Pedro Almendros 2
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.orglett.0c03482 Fernando Herrera 1 , Amparo Luna 1 , Pedro Almendros 2
Affiliation
|
The first coupling of α-allenols, sulfur dioxide, and arenediazonium salts is presented. The three-component reaction which is promoted by visible light can be easily accomplished using DABSO as a sulfur dioxide surrogate in the presence of a photoredox catalyst. In this manner, a broad range of electron-rich and electron-deficient aryl substituents are well accommodated in the sulfonylation–rearrangement cascade to afford the 2,2-disubstituted 3-(arylsulfonyl)but-3-enals in reasonable yields. Based on control experiments, a radical mechanism which does imply 1,2-aryl migration has been proposed.
中文翻译:

α-烯丙醇与重氮盐和二氧化硫的偶合在可见光下Ru催化催化3-(芳基磺酰基)but-3-烯醛的合成
提出了α-烯醇,二氧化硫和槟榔重氮盐的首次偶联。在光氧化还原催化剂的存在下,使用DABSO作为二氧化硫替代物可以轻松完成由可见光促进的三组分反应。以这种方式,在磺酰化-重排级联中可以很好地容纳大量的富电子和缺电子的芳基取代基,从而以合理的收率得到2,2-二取代的3-(芳基磺酰基)but-3-烯醛。基于对照实验,提出了暗示1,2-芳基迁移的自由基机理。
更新日期:2020-12-18
中文翻译:

α-烯丙醇与重氮盐和二氧化硫的偶合在可见光下Ru催化催化3-(芳基磺酰基)but-3-烯醛的合成
提出了α-烯醇,二氧化硫和槟榔重氮盐的首次偶联。在光氧化还原催化剂的存在下,使用DABSO作为二氧化硫替代物可以轻松完成由可见光促进的三组分反应。以这种方式,在磺酰化-重排级联中可以很好地容纳大量的富电子和缺电子的芳基取代基,从而以合理的收率得到2,2-二取代的3-(芳基磺酰基)but-3-烯醛。基于对照实验,提出了暗示1,2-芳基迁移的自由基机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号