当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dearomative Migratory Rearrangement of 2-Oxypyridines Enabled by α-Imino Rhodium Carbene
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.orglett.0c03533 Guangyang Xu 1 , Ying Shao 1 , Shengbiao Tang 1 , Qun Chen 1 , Jiangtao Sun 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.orglett.0c03533 Guangyang Xu 1 , Ying Shao 1 , Shengbiao Tang 1 , Qun Chen 1 , Jiangtao Sun 1
Affiliation
|
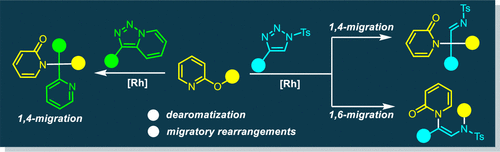
The unprecedented dearomative migratory rearrangement reactions of 2-oxypyridines with N-sulfonyl-1,2,3-triazoles have been developed under rhodium catalysis, providing a reliable and efficient protocol for accessing N-substituted 2-pyridones. These two distinct rearrangements feature the controllable 1,4-migration of a carbonate group from O-to-C as well as the O-to-N 1,6-migration of an acyl group via α-imino rhodium carbene transfer. Moreover, the reaction of pyridotriazoles with 2-oxypyridines delivers the 1,4-migration products in high efficiency.
中文翻译:

α-亚氨基铑卡宾引起的2-氧吡啶的脱芳香族迁移重排
在铑催化下已经开发了2-氧吡啶与N-磺酰基-1,2,3-三唑的空前的脱芳香族迁移重排反应,为获得N-取代的2-吡啶酮提供了可靠而有效的方案。这两个不同的重排的特征是碳酸酯基团从O到C的可控制的1,4-迁移以及酰基通过α-亚氨基铑卡宾转移而从酰基的O到N的1,6-迁移。此外,吡啶并三唑与2-氧吡啶的反应以高效率递送1,4-迁移产物。
更新日期:2020-12-04
中文翻译:

α-亚氨基铑卡宾引起的2-氧吡啶的脱芳香族迁移重排
在铑催化下已经开发了2-氧吡啶与N-磺酰基-1,2,3-三唑的空前的脱芳香族迁移重排反应,为获得N-取代的2-吡啶酮提供了可靠而有效的方案。这两个不同的重排的特征是碳酸酯基团从O到C的可控制的1,4-迁移以及酰基通过α-亚氨基铑卡宾转移而从酰基的O到N的1,6-迁移。此外,吡啶并三唑与2-氧吡啶的反应以高效率递送1,4-迁移产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号