当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
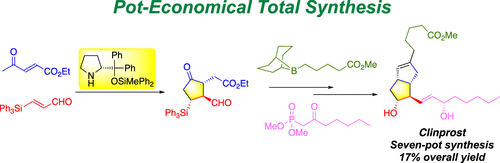
Pot-Economical Total Synthesis of Clinprost
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.orglett.0c03616 Nariyoshi Umekubo 1 , Yujiro Hayashi 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.orglett.0c03616 Nariyoshi Umekubo 1 , Yujiro Hayashi 1
Affiliation
|
The pot-economical synthesis of clinprost is reported, in which the core bicyclo[3.3.0]octenone structure was synthesized by two key steps: an asymmetric domino Michael/Michael reaction catalyzed by diphenylprolinol silyl ether and an intramolecular Horner–Wadsworth–Emmons reaction. The trisubstituted endocyclic alkene was selectively introduced by 1,4-reduction followed by trapping of the generated enolate with Tf2NPh and subsequent utilization of the Suzuki–Miyaura coupling reaction. Chiral, nonracemic clinprost was synthesized in seven pots with a 17% total yield and excellent enantioselectivity.
中文翻译:

克林前列素的全效合成
报道了克林前列素的锅内经济合成,其中核心的双环[3.3.0]辛烯酮结构是通过两个关键步骤合成的:二苯基脯氨醇甲硅烷基醚催化的不对称多米诺Michael / Michael反应和分子内Horner-Wadsworth-Emmons反应。通过1,4-还原选择性地引入三取代的内环烯烃,然后用Tf 2 NPh捕获生成的烯醇化物,随后利用Suzuki-Miyaura偶联反应。在七个罐中合成了手性非外消旋克林前列素,总收率为17%,对映选择性极好。
更新日期:2020-12-04
中文翻译:

克林前列素的全效合成
报道了克林前列素的锅内经济合成,其中核心的双环[3.3.0]辛烯酮结构是通过两个关键步骤合成的:二苯基脯氨醇甲硅烷基醚催化的不对称多米诺Michael / Michael反应和分子内Horner-Wadsworth-Emmons反应。通过1,4-还原选择性地引入三取代的内环烯烃,然后用Tf 2 NPh捕获生成的烯醇化物,随后利用Suzuki-Miyaura偶联反应。在七个罐中合成了手性非外消旋克林前列素,总收率为17%,对映选择性极好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号