当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphine-Catalyzed Sequential [3+3]/Aza-6π-Electrocyclization Reaction of Cross-Conjugated Azatrienes and δ-Sulfonamido-Allenoates
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.orglett.0c03725 Ning Li 1 , You Huang 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.orglett.0c03725 Ning Li 1 , You Huang 1
Affiliation
|
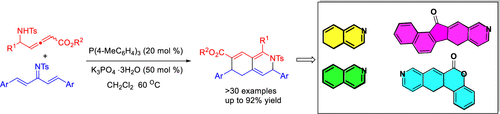
An unprecedented sequential [3+3]/aza-6π-electrocyclization between cross-conjugated azatrienes and δ-sulfonamido-allenoates, catalyzed by phosphine, has been developed, which provides efficient and facile access to highly functionalized tetrahydroisoquinoline derivatives. The products can be easily transformed into (dihydro)isoquinolines and their fused polycyclic compounds. The reactivity of both azatrienes and δ-sulfonamido-allenoates in this text, acting as a five-atom unit, is unique in the phosphine-catalyzed annulations of allenoates.
中文翻译:

膦共轭氮杂三烯与δ-磺酰胺基-脲基甲酸酯的顺序[3 + 3] /Aza-6π-电环化反应
已经开发出了膦催化的交叉共轭氮杂三烯与δ-磺酰胺基-烯丙酸酯之间空前的顺序[3 + 3] /氮杂6π电环化反应,可高效,轻松地获得高度官能化的四氢异喹啉衍生物。产物可以容易地转化为(二氢)异喹啉及其稠合的多环化合物。在本文中,作为五原子单元的氮杂三烯和δ-磺酰胺基-脲基酸酯的反应性在膦基催化的脲基酸酯环中是独特的。
更新日期:2020-12-04
中文翻译:

膦共轭氮杂三烯与δ-磺酰胺基-脲基甲酸酯的顺序[3 + 3] /Aza-6π-电环化反应
已经开发出了膦催化的交叉共轭氮杂三烯与δ-磺酰胺基-烯丙酸酯之间空前的顺序[3 + 3] /氮杂6π电环化反应,可高效,轻松地获得高度官能化的四氢异喹啉衍生物。产物可以容易地转化为(二氢)异喹啉及其稠合的多环化合物。在本文中,作为五原子单元的氮杂三烯和δ-磺酰胺基-脲基酸酯的反应性在膦基催化的脲基酸酯环中是独特的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号