当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective, Organocatalytic Strategy for the Oxazolomycin Core: Formal Synthesis of (+)-Neooxazolomycin
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03511 Christian M Chaheine 1 , Paul T Gladen 1 , Mikail E Abbasov 1 , Daniel Romo 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03511 Christian M Chaheine 1 , Paul T Gladen 1 , Mikail E Abbasov 1 , Daniel Romo 1
Affiliation
|
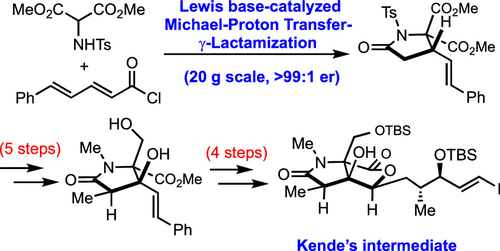
A concise, organocatalytic, enantioselective route to the γ-lactam core of the oxazolomycins was developed. Key steps include a Lewis base-catalyzed, Michael proton transfer–lactamization organocascade, a one-pot N-methylation and diastereoselective α-alkylation, a diastereotopic group-selective reduction, a substrate-directed allylic hydroxylation, and a lanthanide-mediated organolithium addition to append the side chain. A formal synthesis of (+)-neooxazolomycin via interception of a Kende intermediate, accessed in 10 steps (previously 24 steps from α-d-glucose), enabled confirmation of the relative and absolute stereochemistry.
中文翻译:

恶唑霉素核心的对映选择性有机催化策略:(+)-新恶唑霉素的正式合成
开发了一种简洁的、有机催化的、对映选择性的途径,用于恶唑霉素的 γ-内酰胺核心。关键步骤包括路易斯碱催化、迈克尔质子转移-内酰胺化有机级联、一锅 N-甲基化和非对映选择性 α-烷基化、非对映基团选择性还原、底物导向的烯丙基羟基化和镧系元素介导的有机锂加成附加侧链。(+)-neooxazolomycin 通过拦截 Kende 中间体的正式合成,分 10 个步骤(以前从 α- d-葡萄糖24 个步骤)访问,从而能够确认相对和绝对立体化学。
更新日期:2020-12-04
中文翻译:

恶唑霉素核心的对映选择性有机催化策略:(+)-新恶唑霉素的正式合成
开发了一种简洁的、有机催化的、对映选择性的途径,用于恶唑霉素的 γ-内酰胺核心。关键步骤包括路易斯碱催化、迈克尔质子转移-内酰胺化有机级联、一锅 N-甲基化和非对映选择性 α-烷基化、非对映基团选择性还原、底物导向的烯丙基羟基化和镧系元素介导的有机锂加成附加侧链。(+)-neooxazolomycin 通过拦截 Kende 中间体的正式合成,分 10 个步骤(以前从 α- d-葡萄糖24 个步骤)访问,从而能够确认相对和绝对立体化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号