当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Stereoselective Aza-Wacker–Heck Cyclization: One-Pot Stepwise Strategy toward Tetracyclic Fused Heterocycles
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03552 Rong-Shiow Tang, Li-Yuan Chen, Chin-Hung Lai, Ta-Hsien Chuang
Organic Letters ( IF 4.9 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03552 Rong-Shiow Tang, Li-Yuan Chen, Chin-Hung Lai, Ta-Hsien Chuang
|
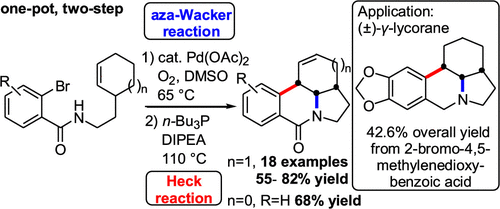
Palladium-catalyzed intramolecular tandem cyclization reactions were conducted for the synthesis of densely cis/cis-fused aza-tetracyclic structures. The process involved a palladium(II)-catalyzed aerobic aza-Wacker reaction, followed by a palladium(0)-catalyzed Heck reaction. The effects of the solvent and benzene substitution pattern on the one-pot, two-step cascade reaction were studied systematically, and a probable mechanism was proposed. Strained pentahydrobenzo[f]cyclopenta[hi]indolizin-6-one and racemic γ-lycorane can also be synthesized rapidly using this palladium-catalyzed aza-Wacker–Heck cyclization reaction.
中文翻译:

钯催化立体选择性Aza-Wacker-Heck环化:四环融合杂环的一锅逐步策略
进行了钯催化的分子内串联环化反应,以合成致密的顺式/顺式稠合的氮杂四环结构。该过程涉及钯(II)催化的好氧aza-Wacker反应,然后是钯(0)催化的Heck反应。系统地研究了溶剂和苯的取代方式对一锅两步级联反应的影响,并提出了可能的机理。使用钯催化的aza-Wacker-Heck环化反应,也可以快速合成应变的五氢苯并[ f ]环戊基[ hi ]吲哚并嗪-6-和外消旋的γ-二十烷。
更新日期:2020-12-04
中文翻译:

钯催化立体选择性Aza-Wacker-Heck环化:四环融合杂环的一锅逐步策略
进行了钯催化的分子内串联环化反应,以合成致密的顺式/顺式稠合的氮杂四环结构。该过程涉及钯(II)催化的好氧aza-Wacker反应,然后是钯(0)催化的Heck反应。系统地研究了溶剂和苯的取代方式对一锅两步级联反应的影响,并提出了可能的机理。使用钯催化的aza-Wacker-Heck环化反应,也可以快速合成应变的五氢苯并[ f ]环戊基[ hi ]吲哚并嗪-6-和外消旋的γ-二十烷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号