Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Transition from the Interdigitated to Bilayer Membrane of a Cationic Surfactant Induced by Addition of Hydrophobic Molecules
Langmuir ( IF 3.7 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.langmuir.0c02609 Mafumi Hishida 1 , Naofumi Shimokawa 2 , Yuki Okubo 1 , Shun Taguchi 1 , Yasuhisa Yamamura 1 , Kazuya Saito 1
Langmuir ( IF 3.7 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.langmuir.0c02609 Mafumi Hishida 1 , Naofumi Shimokawa 2 , Yuki Okubo 1 , Shun Taguchi 1 , Yasuhisa Yamamura 1 , Kazuya Saito 1
Affiliation
|
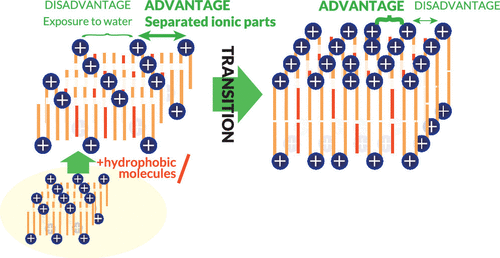
Although the transition between a bilayer and an interdigitated membrane of a surfactant and lipid has been widely known for long, its mechanism remains unclear. This study reveals the transition mechanism of a cationic surfactant, dioctadecyldimethylammonium chloride (DODAC), through experiments and theoretical calculations. Experimentally, the transition from the interdigitated to bilayer structure in the gel phase of DODAC is found to be induced by adding hydrophobic molecules such as n-alkane and its derivatives. Further addition induces a different transition to another bilayer phase. Our theory, considering the competition of the electrostatic interaction between cationic headgroups and the hydrophobic interaction emerging at the alkyl-chain ends exposed to water, reproduces these two phase transitions. In addition, changes in alkyl-chain packing in the membranes at these transitions are reproduced. The underlying mechanism is that the interdigitated membrane is formed at a small additive content due to electrostatic repulsion. As the energetic disadvantage with respect to the hydrophobic interaction becomes dominant as the content increases, the transition to the bilayer occurs at a specific content. The bilayer–bilayer transition at a higher content is induced by the change in the balance of these interactions. Based on a similar concept, we suggest the mechanism of the additive-induced bilayer-interdigitated transition of phospholipids, i.e., neutrally charged (zwitterionic) surfactants.
中文翻译:

疏水性分子的加入引起阳离子表面活性剂从叉指膜到双层膜的相变
尽管很早就已经知道表面活性剂和脂质的双层膜和指状膜之间的过渡,但是其机理仍不清楚。这项研究通过实验和理论计算揭示了阳离子表面活性剂二十八烷基二甲基氯化铵(DODAC)的转变机理。实验上,发现DODAC凝胶相从叉指状结构转变为双层结构是通过添加疏水分子(例如n-烷烃及其衍生物。进一步的添加引起向另一双层相的不同转变。我们的理论考虑了阳离子头基之间的静电相互作用与暴露于水的烷基链末端出现的疏水相互作用之间的竞争,从而再现了这两个相变。另外,在这些转变处,膜中烷基链堆积的变化得以再现。潜在的机理是指叉膜由于静电排斥而以少量添加剂形成。随着关于疏水相互作用的高能劣势随着含量的增加而占主导地位,到双层的转变在特定的含量下发生。这些相互作用之间的平衡变化引起了更高含量的双层-双层转变。
更新日期:2020-12-08
中文翻译:

疏水性分子的加入引起阳离子表面活性剂从叉指膜到双层膜的相变
尽管很早就已经知道表面活性剂和脂质的双层膜和指状膜之间的过渡,但是其机理仍不清楚。这项研究通过实验和理论计算揭示了阳离子表面活性剂二十八烷基二甲基氯化铵(DODAC)的转变机理。实验上,发现DODAC凝胶相从叉指状结构转变为双层结构是通过添加疏水分子(例如n-烷烃及其衍生物。进一步的添加引起向另一双层相的不同转变。我们的理论考虑了阳离子头基之间的静电相互作用与暴露于水的烷基链末端出现的疏水相互作用之间的竞争,从而再现了这两个相变。另外,在这些转变处,膜中烷基链堆积的变化得以再现。潜在的机理是指叉膜由于静电排斥而以少量添加剂形成。随着关于疏水相互作用的高能劣势随着含量的增加而占主导地位,到双层的转变在特定的含量下发生。这些相互作用之间的平衡变化引起了更高含量的双层-双层转变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号