当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Portfolio Targeting Strategy To Realize the Assembly and Membrane Fusion-Mediated Delivery of Gold Nanoparticles to Mitochondria for Enhanced NIR Photothermal Therapies
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.bioconjchem.0c00518 Peng Ning 1 , Liqun Huang 2 , Yuchen Bao 1 , Yingjie Fu 3 , Chang Xu 1 , Yajing Shen 1 , Xiang Zhou 3 , Xiaofei Wen 2 , Yu Cheng 1 , Yao Qin 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.bioconjchem.0c00518 Peng Ning 1 , Liqun Huang 2 , Yuchen Bao 1 , Yingjie Fu 3 , Chang Xu 1 , Yajing Shen 1 , Xiang Zhou 3 , Xiaofei Wen 2 , Yu Cheng 1 , Yao Qin 1
Affiliation
|
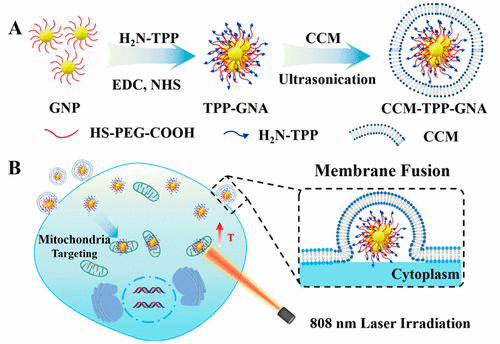
Targeting mitochondria has always been a challenging goal for therapeutic nanoparticle agents due to their heterotypic features and size, which usually lead to a lysosome/endosome endocytosis pathway. To overcome this limitation, in this work, a portfolio targeting strategy combining a small targeting molecule with a biomembrane was developed. Modification of small targeting molecule H2N-TPP on gold nanoparticles (GNPs) could not only facilitate the mitochondrial targeting but could also induce gold nanoparticle assembly. Therefore, the GNPs were endowed with good absorption and photothermal conversion abilities in the near-infrared (NIR) region. Meanwhile, a biomimetic strategy was adopted by wrapping the gold nanoparticle assembly (GNA) with cancer cell membranes (CCMs), which helped the GNA enter the prostatic cancer cell via a homotypic membrane-fusion process to avoid being trapped in endosomes/lysosomes. Thereafter, the GNA remaining in the cytoplasm could reach mitochondria more efficiently via guidance from H2N-TPP molecules. This “biomembrane–small molecule” combination targeting process was evidenced by fluorescence microscopy, and the highly efficient photothermal ablation of prostatic tumors in vivo was demonstrated. This portfolio targeting strategy could be extended to various nanodrugs/agents to realize an accurate subcellular targeting efficiency for cancer treatments or cell detections.
中文翻译:

资产组合目标战略,以实现组装和膜融合介导的金纳米颗粒向线粒体的递送,以增强NIR光热疗法
靶向线粒体由于其异型特征和大小通常一直是溶酶体/内体内吞途径,因此一直是治疗性纳米颗粒药物的挑战性目标。为了克服这一局限性,在这项工作中,开发了将小分子靶向分子与生物膜结合起来的投资组合靶向策略。小分子靶向分子H 2的修饰金纳米粒子(GNP)上的N-TPP不仅可以促进线粒体靶向,而且还可以诱导金纳米粒子组装。因此,GNP在近红外(NIR)区域具有良好的吸收和光热转化能力。同时,采用了仿生策略,将金纳米颗粒组件(GNA)包裹在癌细胞膜(CCM)上,这有助于GNA通过同型膜融合过程进入前列腺癌细胞,从而避免陷入内体/溶酶体中。此后,通过H 2的引导,保留在细胞质中的GNA可以更有效地到达线粒体N-TPP分子。荧光显微镜证明了这种“双膜-小分子”组合靶向过程,并证明了体内前列腺肿瘤的高效光热消融。该投资组合靶向策略可以扩展到各种纳米药物/试剂,以实现用于癌症治疗或细胞检测的准确的亚细胞靶向效率。
更新日期:2020-12-16
中文翻译:

资产组合目标战略,以实现组装和膜融合介导的金纳米颗粒向线粒体的递送,以增强NIR光热疗法
靶向线粒体由于其异型特征和大小通常一直是溶酶体/内体内吞途径,因此一直是治疗性纳米颗粒药物的挑战性目标。为了克服这一局限性,在这项工作中,开发了将小分子靶向分子与生物膜结合起来的投资组合靶向策略。小分子靶向分子H 2的修饰金纳米粒子(GNP)上的N-TPP不仅可以促进线粒体靶向,而且还可以诱导金纳米粒子组装。因此,GNP在近红外(NIR)区域具有良好的吸收和光热转化能力。同时,采用了仿生策略,将金纳米颗粒组件(GNA)包裹在癌细胞膜(CCM)上,这有助于GNA通过同型膜融合过程进入前列腺癌细胞,从而避免陷入内体/溶酶体中。此后,通过H 2的引导,保留在细胞质中的GNA可以更有效地到达线粒体N-TPP分子。荧光显微镜证明了这种“双膜-小分子”组合靶向过程,并证明了体内前列腺肿瘤的高效光热消融。该投资组合靶向策略可以扩展到各种纳米药物/试剂,以实现用于癌症治疗或细胞检测的准确的亚细胞靶向效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号