当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Determinants of Flavin Dynamics in a Class B Monooxygenase
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.biochem.0c00783 Ashley C. Campbell 1 , Reeder Robinson 2 , Didier Mena-Aguilar 2 , Pablo Sobrado 2 , John J. Tanner 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.biochem.0c00783 Ashley C. Campbell 1 , Reeder Robinson 2 , Didier Mena-Aguilar 2 , Pablo Sobrado 2 , John J. Tanner 1, 3
Affiliation
|
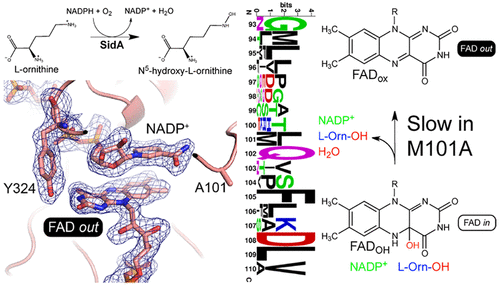
The ornithine hydroxylase known as SidA is a class B flavin monooxygenase that catalyzes the first step in the biosynthesis of hydroxamate-containing siderophores in Aspergillus fumigatus. Crystallographic studies of SidA revealed that the FAD undergoes dramatic conformational changes between out and in states during the catalytic cycle. We sought insight into the origins and purpose of flavin motion in class B monooxygenases by probing the function of Met101, a residue that contacts the pyrimidine ring of the in FAD. Steady-state kinetic measurements showed that the mutant variant M101A has a 25-fold lower turnover number. Pre-steady-state kinetic measurements, pH profiles, and solvent kinetic isotope effect measurements were used to isolate the microscopic step that is responsible for the reduced steady-state activity. The data are consistent with a bottleneck in the final step of the mechanism, which involves flavin dehydration and the release of hydroxy-l-ornithine and NADP+. Crystal structures were determined for M101A in the resting state and complexed with NADP+. The resting enzyme structure is similar to that of wild-type SidA, consistent with M101A exhibiting normal kinetics for flavin reduction by NADPH and wild-type affinity for NADPH. In contrast, the structure of the M101A–NADP+ complex unexpectedly shows the FAD adopting the out conformation and may represent a stalled conformation that is responsible for the slow kinetics. Altogether, our data support a previous proposal that one purpose of the FAD conformational change from in to out in class B flavin monooxygenases is to eject spent NADP+ in preparation for a new catalytic cycle.
中文翻译:

B类单加氧酶中黄素动力学的结构决定因素
称为SidA的鸟氨酸羟化酶是B类黄素单加氧酶,可催化烟曲霉中含异羟肟酸铁载体生物合成的第一步。SidA的晶体学研究表明,FAD在催化循环过程中发生了从外到内的剧烈构象变化。我们通过探测Met101的功能来探索B类单加氧酶中黄素运动的起源和目的,Met101是一个残基,与嘧啶的嘧啶环接触。FAD。稳态动力学测量表明,突变体M101A的周转率降低了25倍。稳态前动力学测量,pH分布图和溶剂动力学同位素效应测量用于隔离导致稳态活性降低的微观步骤。数据与该机制最后一步的瓶颈相一致,该瓶颈涉及黄素脱水以及羟基-1-鸟氨酸和NADP +的释放。确定了M101A在静止状态下的晶体结构,并与NADP +络合。静止的酶结构与野生型SidA相似,与M101A表现出通过NADPH还原黄素的正常动力学和对NADPH的野生型亲和力一致。相反,M101A–NADP +配合物的结构出乎意料地表明FAD采用了构象,可能代表了失速构象,这是动力学缓慢的原因。总之,我们的数据支持先前的提议,即从FAD构象变化的一个目的在以出在B类黄素单加氧酶是花NADP弹出+,准备一个新的催化循环。
更新日期:2020-12-08
中文翻译:

B类单加氧酶中黄素动力学的结构决定因素
称为SidA的鸟氨酸羟化酶是B类黄素单加氧酶,可催化烟曲霉中含异羟肟酸铁载体生物合成的第一步。SidA的晶体学研究表明,FAD在催化循环过程中发生了从外到内的剧烈构象变化。我们通过探测Met101的功能来探索B类单加氧酶中黄素运动的起源和目的,Met101是一个残基,与嘧啶的嘧啶环接触。FAD。稳态动力学测量表明,突变体M101A的周转率降低了25倍。稳态前动力学测量,pH分布图和溶剂动力学同位素效应测量用于隔离导致稳态活性降低的微观步骤。数据与该机制最后一步的瓶颈相一致,该瓶颈涉及黄素脱水以及羟基-1-鸟氨酸和NADP +的释放。确定了M101A在静止状态下的晶体结构,并与NADP +络合。静止的酶结构与野生型SidA相似,与M101A表现出通过NADPH还原黄素的正常动力学和对NADPH的野生型亲和力一致。相反,M101A–NADP +配合物的结构出乎意料地表明FAD采用了构象,可能代表了失速构象,这是动力学缓慢的原因。总之,我们的数据支持先前的提议,即从FAD构象变化的一个目的在以出在B类黄素单加氧酶是花NADP弹出+,准备一个新的催化循环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号