当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recombination of 2Fe-2S Ferredoxins Reveals Differences in the Inheritance of Thermostability and Midpoint Potential
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-23 , DOI: 10.1021/acssynbio.0c00303 Ian J. Campbell 1 , Dimithree Kahanda 1 , Joshua T. Atkinson 1 , Othneil Noble Sparks 1 , Jinyoung Kim 1 , Chia-Ping Tseng 2 , Rafael Verduzco 2 , George N. Bennett 1, 2 , Jonathan J. Silberg 1, 2, 3
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-23 , DOI: 10.1021/acssynbio.0c00303 Ian J. Campbell 1 , Dimithree Kahanda 1 , Joshua T. Atkinson 1 , Othneil Noble Sparks 1 , Jinyoung Kim 1 , Chia-Ping Tseng 2 , Rafael Verduzco 2 , George N. Bennett 1, 2 , Jonathan J. Silberg 1, 2, 3
Affiliation
|
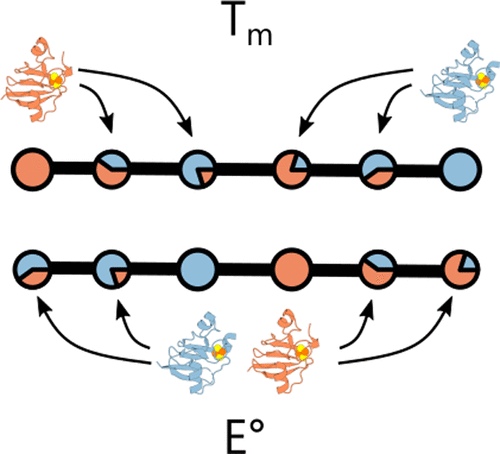
Recombination can be used in the laboratory to overcome component limitations in synthetic biology by creating enzymes that exhibit distinct activities and stabilities from native proteins. To investigate how recombination affects the properties of an oxidoreductase that transfers electrons in cells, we created ferredoxin (Fd) chimeras by recombining distantly related cyanobacterial and cyanomyophage Fds (53% identity) that present similar midpoint potentials but distinct thermostabilities. Fd chimeras having a wide range of amino acid substitutions retained the ability to coordinate an iron–sulfur cluster, although their thermostabilities varied with the fraction of residues inherited from each parent. The midpoint potentials of chimeric Fds also varied. However, all of the synthetic Fds exhibited midpoint potentials outside of the parental protein range. Each of the chimeric Fds could also support electron transfer between Fd-NADP reductase and sulfite reductase in Escherichia coli, although the chimeric Fds varied in the expression required for similar levels of cellular electron transfer. These results show how Fds can be diversified through recombination and reveal differences in the inheritance of thermostability and electrochemical properties. Furthermore, they illustrate how electron transfer efficiencies of chimeric Fds can be rapidly evaluated using a synthetic metabolic pathway.
中文翻译:

2Fe-2S铁氧还蛋白的重组揭示了热稳定性和中点电位的遗传差异
重组可用于实验室中,通过创造出与天然蛋白质具有不同活性和稳定性的酶来克服合成生物学中的成分限制。为了研究重组如何影响在细胞中转移电子的氧化还原酶的性质,我们通过重组具有相似中点电位但具有不同热稳定性的远缘相关的蓝细菌和蓝噬菌体Fds(53%相同)来创建铁氧还蛋白(Fd)嵌合体。尽管氨基酸的热稳定性随从每个亲本继承的残基的分数而变化,但具有广泛氨基酸取代的Fd嵌合体仍具有协调铁硫簇的能力。嵌合Fds的中点电位也变化。然而,所有合成的Fds都显示出超出亲本蛋白质范围的中点电位。每个嵌合Fds还可以支持Fd-NADP还原酶和亚硫酸盐还原酶之间的电子转移。大肠杆菌,尽管嵌合Fds在表达相似水平的细胞电子转移所需的表达上有所不同。这些结果显示了Fds如何通过重组而多样化,并揭示了热稳定性和电化学性质的遗传差异。此外,它们说明了如何使用合成代谢途径快速评估嵌合Fds的电子转移效率。
更新日期:2020-12-18
中文翻译:

2Fe-2S铁氧还蛋白的重组揭示了热稳定性和中点电位的遗传差异
重组可用于实验室中,通过创造出与天然蛋白质具有不同活性和稳定性的酶来克服合成生物学中的成分限制。为了研究重组如何影响在细胞中转移电子的氧化还原酶的性质,我们通过重组具有相似中点电位但具有不同热稳定性的远缘相关的蓝细菌和蓝噬菌体Fds(53%相同)来创建铁氧还蛋白(Fd)嵌合体。尽管氨基酸的热稳定性随从每个亲本继承的残基的分数而变化,但具有广泛氨基酸取代的Fd嵌合体仍具有协调铁硫簇的能力。嵌合Fds的中点电位也变化。然而,所有合成的Fds都显示出超出亲本蛋白质范围的中点电位。每个嵌合Fds还可以支持Fd-NADP还原酶和亚硫酸盐还原酶之间的电子转移。大肠杆菌,尽管嵌合Fds在表达相似水平的细胞电子转移所需的表达上有所不同。这些结果显示了Fds如何通过重组而多样化,并揭示了热稳定性和电化学性质的遗传差异。此外,它们说明了如何使用合成代谢途径快速评估嵌合Fds的电子转移效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号