当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cellular Proliferation, Self-Assembly, and Modulation of Signaling Pathways in Silk Fibroin Gelatin-Based 3D Bioprinted Constructs
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-11-24 , DOI: 10.1021/acsabm.0c01252 Juhi Chakraborty 1 , Sourabh Ghosh 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2020-11-24 , DOI: 10.1021/acsabm.0c01252 Juhi Chakraborty 1 , Sourabh Ghosh 1
Affiliation
|
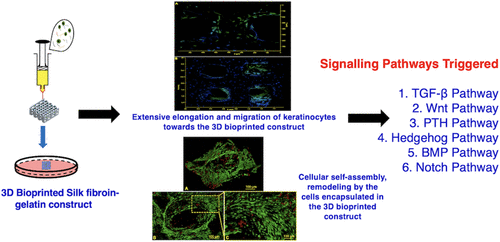
Three-dimensional (3D) bioprinting is a highly innovative and promising technology to render precise positioning of biologics together with living cells and extracellular matrix (ECM) constituents. In spite of such enthralling potential, the fabrication of a clinically relevant engineered tissue is quite challenging. A constellation of factors simulating the complex architecture of the native tissue, selection of the “ideal bioink”, optimization of the biochemical, mechanical, and topographical functions of the cell-laden printed construct, cellular differentiation, their self-assembly, and remodeling into the desired lineage postprinting present major complications. Keeping this in view, we have attempted to highlight the use of silk fibroin (SF) protein from Bombyx mori silkworm as a promising biomaterial of choice for the formulation of bioink owing to its distinct characteristics involving rheology behavior, self-supporting filamentous extrusion, and a suitable biomaterial to achieve resolution printing. Further, we have elaborated on how SF gelatin bioink can in specific regulate the cellular differentiation pathway of progenitor cells, the mechanism of cellular self-assembly, cell migration, matrix remodeling, and self-orientation, leading to the desired tissue-specific construct. How features of bioink and fabrication design aspects can induce in vitro tissue patterning and anatomically relevant tissue organization have also been explored in this review. Importantly, we have tried to shift the understanding of bioprinted tissue regeneration from a cell-proliferation-centric and gene-expression-centric point of view to the complex role of the microenvironment present within the bioprinted constructs. We believe that shedding light on these factors would help in achieving the so-called “ideal 3D bioprinted construct” to meet the shortages of high-quality donor tissues for the regeneration of the damaged and diseased ones.
中文翻译:

基于丝素蛋白明胶的 3D 生物打印构建体中的细胞增殖、自组装和信号通路调节
三维 (3D) 生物打印是一种高度创新和有前途的技术,可以将生物制剂与活细胞和细胞外基质 (ECM) 成分一起精确定位。尽管具有如此迷人的潜力,但临床相关的工程组织的制造仍相当具有挑战性。模拟天然组织复杂结构的一系列因素,“理想生物墨水”的选择,载有细胞的印刷结构的生化、机械和地形功能的优化、细胞分化、它们的自组装和重塑所需的谱系印后存在重大并发症。考虑到这一点,我们试图强调使用来自家蚕的丝素蛋白 (SF)蚕是一种很有前途的生物墨水配方的首选生物材料,因为它具有流变行为、自支撑丝状挤出和适合实现分辨率打印的生物材料的独特特性。此外,我们还详细阐述了 SF 明胶生物墨水如何特异性调节祖细胞的细胞分化途径、细胞自组装机制、细胞迁移、基质重塑和自我定向,从而产生所需的组织特异性构建体。生物墨水和制造设计方面的特征如何在体外诱导本综述还探讨了组织模式和解剖学相关的组织组织。重要的是,我们试图将对生物打印组织再生的理解从以细胞增殖和基因表达为中心的观点转变为生物打印结构中存在的微环境的复杂作用。我们相信,阐明这些因素将有助于实现所谓的“理想的 3D 生物打印结构”,以满足受损和患病组织再生所需的高质量供体组织的短缺。
更新日期:2020-12-21
中文翻译:

基于丝素蛋白明胶的 3D 生物打印构建体中的细胞增殖、自组装和信号通路调节
三维 (3D) 生物打印是一种高度创新和有前途的技术,可以将生物制剂与活细胞和细胞外基质 (ECM) 成分一起精确定位。尽管具有如此迷人的潜力,但临床相关的工程组织的制造仍相当具有挑战性。模拟天然组织复杂结构的一系列因素,“理想生物墨水”的选择,载有细胞的印刷结构的生化、机械和地形功能的优化、细胞分化、它们的自组装和重塑所需的谱系印后存在重大并发症。考虑到这一点,我们试图强调使用来自家蚕的丝素蛋白 (SF)蚕是一种很有前途的生物墨水配方的首选生物材料,因为它具有流变行为、自支撑丝状挤出和适合实现分辨率打印的生物材料的独特特性。此外,我们还详细阐述了 SF 明胶生物墨水如何特异性调节祖细胞的细胞分化途径、细胞自组装机制、细胞迁移、基质重塑和自我定向,从而产生所需的组织特异性构建体。生物墨水和制造设计方面的特征如何在体外诱导本综述还探讨了组织模式和解剖学相关的组织组织。重要的是,我们试图将对生物打印组织再生的理解从以细胞增殖和基因表达为中心的观点转变为生物打印结构中存在的微环境的复杂作用。我们相信,阐明这些因素将有助于实现所谓的“理想的 3D 生物打印结构”,以满足受损和患病组织再生所需的高质量供体组织的短缺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号