Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homochiral bifunctional L‐prolinamide‐ and L‐bis‐prolinamide‐catalyzed asymmetric aldol reactions performed in wet solvent‐free conditions
Chirality ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1002/chir.23283 Gabriela Huelgas 1 , Ratnasamy Somanathan 2 , Julio M Hernández Pérez 3 , Haydee Rojas Cabrera 1 , Maximiliano de la Higuera Macías 1 , Alejandra Domínguez-Huerta 1 , Rocío Sabala 1 , Cecilia Anaya de Parrodi 1
Chirality ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1002/chir.23283 Gabriela Huelgas 1 , Ratnasamy Somanathan 2 , Julio M Hernández Pérez 3 , Haydee Rojas Cabrera 1 , Maximiliano de la Higuera Macías 1 , Alejandra Domínguez-Huerta 1 , Rocío Sabala 1 , Cecilia Anaya de Parrodi 1
Affiliation
|
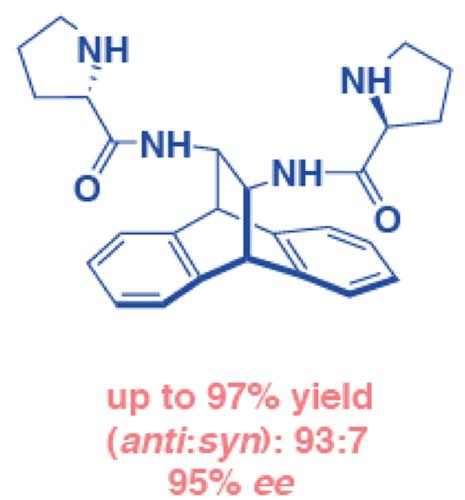
In this study, the novel bifunctional homochiral thiourea‐L‐prolinamides 1–4, tertiary amino‐L‐prolinamide 5, and bis‐L‐prolinamides 6 and 7 were prepared from enantiomerically pure (11R,12R)‐11,12‐diamino‐9,10‐dihydro‐9,10‐ethanoanthracene 8 and (11S,12S)‐11,12‐diamino‐9,10‐dihydro‐9,10‐ethanoanthracene ent‐8. Highly enantioselective and diastereoselective aldolic intermolecular reactions (up to 95% enantiomeric excess, 93:7 anti/syn) between aliphatic ketones (20 equiv) and a range of aromatic aldehydes (1 equiv) were successfully carried out in the presence of water (10 equiv) and monochloroacetic acid (10 mol%), solvent‐free conditions, at room temperature over 24 h using organocatalysts 1–7 (5 mol%). Stereoselective induction using density functional theory–based methods was consistent with the experimental data.
中文翻译:

在湿无溶剂条件下进行的同手性双功能 L-脯氨酰胺和 L-双-脯氨酰胺催化的不对称醛醇反应
在本研究中,新型双功能单手性硫脲-L-脯氨酰胺1-4、叔氨基-L-脯氨酰胺5和双-L-脯氨酰胺6和7由对映体纯的 (11 R ,12 R )-11,12 制备-diamino-9,10-dihydro-9,10-ethanoanthracene 8和 (11 S ,12 S )-11,12-diamino-9,10-dihydro-9,10-ethanoanthracene ent -8。高度对映选择性和非对映选择性醛醇分子间反应(高达 95% 对映体过量, 93:7抗/顺式)脂肪族酮(20当量)和一系列芳族醛的之间(1个当量)被成功地在水(10当量)和氯乙酸(10摩尔%),无溶剂存在的条件下经进行,在室温下使用有机催化剂1–7 (5 mol%) 24 小时。使用基于密度泛函理论的方法立体选择性诱导与实验数据一致。
更新日期:2020-12-12
中文翻译:

在湿无溶剂条件下进行的同手性双功能 L-脯氨酰胺和 L-双-脯氨酰胺催化的不对称醛醇反应
在本研究中,新型双功能单手性硫脲-L-脯氨酰胺1-4、叔氨基-L-脯氨酰胺5和双-L-脯氨酰胺6和7由对映体纯的 (11 R ,12 R )-11,12 制备-diamino-9,10-dihydro-9,10-ethanoanthracene 8和 (11 S ,12 S )-11,12-diamino-9,10-dihydro-9,10-ethanoanthracene ent -8。高度对映选择性和非对映选择性醛醇分子间反应(高达 95% 对映体过量, 93:7抗/顺式)脂肪族酮(20当量)和一系列芳族醛的之间(1个当量)被成功地在水(10当量)和氯乙酸(10摩尔%),无溶剂存在的条件下经进行,在室温下使用有机催化剂1–7 (5 mol%) 24 小时。使用基于密度泛函理论的方法立体选择性诱导与实验数据一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号