当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asn‐linked N–acetylglucosamine of the amylin receptor 2 extracellular domain enhances peptide ligand affinity
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-11-23 , DOI: 10.1002/2211-5463.13042 Sangmin Lee 1
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-11-23 , DOI: 10.1002/2211-5463.13042 Sangmin Lee 1
Affiliation
|
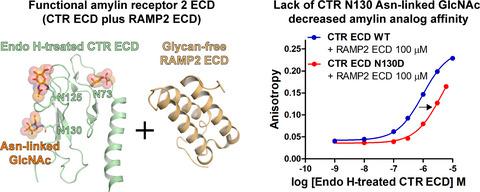
The calcitonin receptor (CTR) has a large extracellular domain (ECD) with multiple N‐glycosylation sites. An asparagine (Asn)‐linked N‐acetylglucosamine (GlcNAc) of CTR ECD N130 was previously reported to enhance peptide hormone binding affinity for CTR ECD. CTR forms a complex with an accessory protein RAMP, and the RAMP:CTR complex gains affinity for peptide hormone amylin as the amylin receptor (AMY). Although N‐glycosylation of AMY ECD was reported to enhance peptide hormone affinity, it remains underexplored which N‐glycosites of AMY ECD are responsible for peptide affinity enhancement and it is unclear whether an Asn‐linked GlcNAc of the N‐glycosites plays a critical role. Here, I investigated the role of the Asn‐linked GlcNAc of CTR N130 in the affinity of an antagonistic amylin analog (AC413) for AMY2 ECD (the RAMP2 ECD:CTR ECD complex). I used Endo H‐treated CTR ECD in which N‐glycans were trimmed to an Asn‐linked GlcNAc on each of the N‐glycosites. I incubated Endo H‐treated CTR ECD with excess of glycan‐free RAMP2 ECD to produce the RAMP2 ECD:CTR ECD complex. Using this coincubation system, I found that the RAMP2 ECD complex with Endo H‐treated CTR ECD with N130D mutation showed a fourfold decrease in AC413 affinity compared with the RAMP2 ECD complex with Endo H‐treated CTR ECD WT. In contrast, RAMP2 ECD N‐glycosylation did not affect peptide binding affinity. These results indicate that the Asn‐linked GlcNAc of CTR N130 is an important peptide affinity enhancer for AMY2 ECD and reveals a significant role of the Asn‐linked GlcNAc in AMY2 function.
中文翻译:

胰淀素受体 2 胞外结构域的 Asn 连接的 N-乙酰氨基葡萄糖增强肽配体亲和力
降钙素受体 (CTR) 具有带有多个N-糖基化位点的大细胞外结构域 (ECD)。先前报道了 CTR ECD N130的天冬酰胺 (Asn) 连接的N-乙酰氨基葡萄糖 (GlcNAc) 可增强肽激素对 CTR ECD 的结合亲和力。CTR 与辅助蛋白 RAMP 形成复合物,RAMP:CTR 复合物对肽激素糊精受体 (AMY) 具有亲和力。尽管据报道 AMY ECD 的N-糖基化可增强肽激素的亲和力,但 AMY ECD 的哪些N-糖苷对肽亲和力增强的作用仍未得到充分探索,并且尚不清楚N的 Asn 连接的 GlcNAc糖苷类起着至关重要的作用。在这里,我研究了 CTR N130 的 Asn 连接的 GlcNAc 在拮抗性胰淀素类似物 (AC413) 对 AMY 2 ECD(RAMP2 ECD:CTR ECD 复合物)的亲和力中的作用。我使用了 Endo H 处理的 CTR ECD,其中N-聚糖被修剪为每个N-糖苷上的 Asn 连接的 GlcNAc。我将 Endo H 处理的 CTR ECD 与过量的无聚糖 RAMP2 ECD 一起孵育,以产生 RAMP2 ECD:CTR ECD 复合物。使用这种共孵育系统,我发现与带有 Endo H 处理的 CTR ECD WT 的 RAMP2 ECD 复合物相比,带有 N130D 突变的 Endo H 处理的 CTR ECD 的 RAMP2 ECD 复合物的 AC413 亲和力降低了四倍。相比之下,RAMP2 ECD N-糖基化不影响肽结合亲和力。这些结果表明,CTR N130 的 Asn 连接的 GlcNAc 是 AMY 2 ECD 的重要肽亲和力增强剂,并揭示了 Asn 连接的 GlcNAc 在 AMY 2功能中的重要作用。
更新日期:2021-01-04
中文翻译:

胰淀素受体 2 胞外结构域的 Asn 连接的 N-乙酰氨基葡萄糖增强肽配体亲和力
降钙素受体 (CTR) 具有带有多个N-糖基化位点的大细胞外结构域 (ECD)。先前报道了 CTR ECD N130的天冬酰胺 (Asn) 连接的N-乙酰氨基葡萄糖 (GlcNAc) 可增强肽激素对 CTR ECD 的结合亲和力。CTR 与辅助蛋白 RAMP 形成复合物,RAMP:CTR 复合物对肽激素糊精受体 (AMY) 具有亲和力。尽管据报道 AMY ECD 的N-糖基化可增强肽激素的亲和力,但 AMY ECD 的哪些N-糖苷对肽亲和力增强的作用仍未得到充分探索,并且尚不清楚N的 Asn 连接的 GlcNAc糖苷类起着至关重要的作用。在这里,我研究了 CTR N130 的 Asn 连接的 GlcNAc 在拮抗性胰淀素类似物 (AC413) 对 AMY 2 ECD(RAMP2 ECD:CTR ECD 复合物)的亲和力中的作用。我使用了 Endo H 处理的 CTR ECD,其中N-聚糖被修剪为每个N-糖苷上的 Asn 连接的 GlcNAc。我将 Endo H 处理的 CTR ECD 与过量的无聚糖 RAMP2 ECD 一起孵育,以产生 RAMP2 ECD:CTR ECD 复合物。使用这种共孵育系统,我发现与带有 Endo H 处理的 CTR ECD WT 的 RAMP2 ECD 复合物相比,带有 N130D 突变的 Endo H 处理的 CTR ECD 的 RAMP2 ECD 复合物的 AC413 亲和力降低了四倍。相比之下,RAMP2 ECD N-糖基化不影响肽结合亲和力。这些结果表明,CTR N130 的 Asn 连接的 GlcNAc 是 AMY 2 ECD 的重要肽亲和力增强剂,并揭示了 Asn 连接的 GlcNAc 在 AMY 2功能中的重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号