当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of the performances of different atomic charge and nonelectrostatic models in the finite‐difference Poisson–Boltzmann approach
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-11-24 , DOI: 10.1002/qua.26560 Dario Vassetti 1 , Frédéric Labat 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-11-24 , DOI: 10.1002/qua.26560 Dario Vassetti 1 , Frédéric Labat 1
Affiliation
|
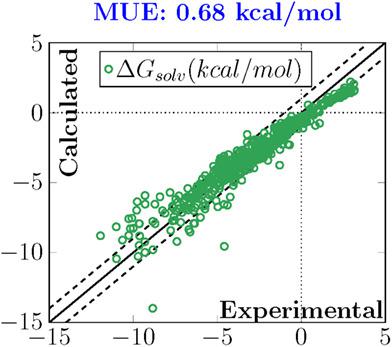
In this work, we investigate the effects of different atomic charge and nonelectrostatic models on the hydration energies of neutral molecules, using an implicit solvation model. The solvation free energy is divided into two main components, the first resulting from a self‐consistent reaction field treatment of the bulk electrostatics obtained by solving the Poisson equation in a finite‐difference (FD) approach where the solute charge density is approximated by atomic charges, the second corresponding to short‐range interactions between the solute and the solvent in the first solvation shell. Five different atomic charge models (Mulliken, Hirshfeld, Hirshfeld‐I, CM5 and its iterative version, CM5‐I) have been considered, both at the Hartree–Fock (HF) and B3LYP levels, with three different basis sets, alongside two nonelectrostatic models including the cavity, dispersion, and solvent structural effects (CDS) model. Averaging over the three considered basis sets, Hirshfeld charges combined to the CDS model led to the lowest mean unsigned error (MUE), with a value of 0.92 kcal/mol with respect to the experimental data. On the other hand, a MUE of 2.02 kcal/mol was obtained with CM5 charges combined to the CDS model, highlighting the low transferability of the original CDS parameters developed for the generalized Born electrostatics to a different electrostatics model. By scaling down the CM5 charges to better balance with the original CDS model, a MUE of 0.68 kcal/mol was however obtained, outlining the delicate balance existing between the electrostatic and nonelectrostatic contributions to the solvation free energy in implicit solvation models.
中文翻译:

有限差分泊松-玻尔兹曼方法评估不同原子电荷和非静电模型的性能
在这项工作中,我们使用隐式溶剂化模型研究了不同原子电荷和非静电模型对中性分子水合能量的影响。溶剂化自由能分为两个主要成分,第一个成分是对本体静电的自洽反应场处理所产生的,该场静电是通过有限差分(FD)方法求解泊松方程获得的,其中溶质电荷密度近似于原子电荷,第二个对应于第一个溶剂化壳中溶质和溶剂之间的短程相互作用。已经考虑了五种不同的原子电荷模型(Mulliken,Hirshfeld,Hirshfeld-I,CM5及其迭代版本CM5-I),并在Hartree-Fock(HF)和B3LYP级别上使用了三种不同的基集,以及两个非静电模型,包括空穴模型,分散模型和溶剂结构效应(CDS)模型。在三个考虑的基集上平均,与CDS模型组合的Hirshfeld电荷导致最低的平均无符号误差(MUE),相对于实验数据值为0.92 kcal / mol。另一方面,通过将CM5电荷结合到CDS模型中,可以得到2.02 kcal / mol的MUE,这突出显示了为广义Born静电学开发的原始CDS参数到其他静电学模型的低转移性。通过按比例缩小CM5电荷以更好地与原始CDS模型保持平衡,可得到0.68 kcal / mol的MUE,概述了隐式溶剂化模型中对溶剂化自由能的静电和非静电作用之间存在的微妙平衡。
更新日期:2020-11-24
中文翻译:

有限差分泊松-玻尔兹曼方法评估不同原子电荷和非静电模型的性能
在这项工作中,我们使用隐式溶剂化模型研究了不同原子电荷和非静电模型对中性分子水合能量的影响。溶剂化自由能分为两个主要成分,第一个成分是对本体静电的自洽反应场处理所产生的,该场静电是通过有限差分(FD)方法求解泊松方程获得的,其中溶质电荷密度近似于原子电荷,第二个对应于第一个溶剂化壳中溶质和溶剂之间的短程相互作用。已经考虑了五种不同的原子电荷模型(Mulliken,Hirshfeld,Hirshfeld-I,CM5及其迭代版本CM5-I),并在Hartree-Fock(HF)和B3LYP级别上使用了三种不同的基集,以及两个非静电模型,包括空穴模型,分散模型和溶剂结构效应(CDS)模型。在三个考虑的基集上平均,与CDS模型组合的Hirshfeld电荷导致最低的平均无符号误差(MUE),相对于实验数据值为0.92 kcal / mol。另一方面,通过将CM5电荷结合到CDS模型中,可以得到2.02 kcal / mol的MUE,这突出显示了为广义Born静电学开发的原始CDS参数到其他静电学模型的低转移性。通过按比例缩小CM5电荷以更好地与原始CDS模型保持平衡,可得到0.68 kcal / mol的MUE,概述了隐式溶剂化模型中对溶剂化自由能的静电和非静电作用之间存在的微妙平衡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号