当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(III)‐Catalyzed Alkylation of 2‐Arylquinazolin‐4(3H)‐ones with Cyclopropanols by Directing C‐H Activation and Ring Opening at Ambient Temperature
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1002/ajoc.202000459 Mu‐Wang Chen 1 , Minhao Lou 1 , Zhihong Deng 1 , Qin Yang 1 , Yiyuan Peng 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1002/ajoc.202000459 Mu‐Wang Chen 1 , Minhao Lou 1 , Zhihong Deng 1 , Qin Yang 1 , Yiyuan Peng 1
Affiliation
|
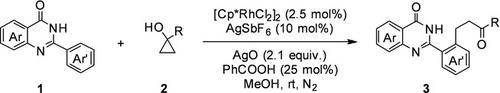
An efficient method was developed for the alkylation of 2‐arylquinazolin‐4(3H)‐ones with cyclopropanols. The desired products β‐aryl ketones bearing the quinazolin‐4(3H)‐one scaffold were synthesized by Rh(III)‐catalyzed C−H activation of arenes and C−C cleavage of cyclopropanols. This method has a wide range of benzyl/phenyl substrate applicability and provides a theoretical guidance for our research to study quinazoline compounds.
中文翻译:

铑(III)在环境温度下指导C-H活化和开环反应,催化2-芳基喹唑啉-4(3H)-1与环丙醇的烷基化
开发了一种有效的方法,用于将2-芳基喹唑啉-4(3H)-one与环丙醇烷基化。通过Rh(III)催化的芳烃的CH活化和环丙醇的CC裂解合成了带有喹唑啉-4(3H)-1骨架的所需产物β-芳基酮。该方法具有广泛的苄基/苯基底物适用性,为我们研究喹唑啉化合物的研究提供了理论指导。
更新日期:2021-01-18
中文翻译:

铑(III)在环境温度下指导C-H活化和开环反应,催化2-芳基喹唑啉-4(3H)-1与环丙醇的烷基化
开发了一种有效的方法,用于将2-芳基喹唑啉-4(3H)-one与环丙醇烷基化。通过Rh(III)催化的芳烃的CH活化和环丙醇的CC裂解合成了带有喹唑啉-4(3H)-1骨架的所需产物β-芳基酮。该方法具有广泛的苄基/苯基底物适用性,为我们研究喹唑啉化合物的研究提供了理论指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号