Structure ( IF 4.4 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.str.2020.11.005 Matthew T Eddy 1 , Bryan T Martin 2 , Kurt Wüthrich 3
|
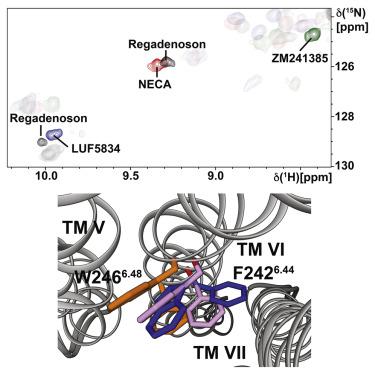
In drug design, G protein-coupled receptor (GPCR) partial agonists enable one to fine-tune receptor output between basal and maximal signaling levels. Here, we add to the structural basis for rationalizing and monitoring partial agonism. NMR spectroscopy of partial agonist complexes of the A2A adenosine receptor (A2AAR) revealed conformations of the P-I-F activation motif that are distinctly different from full agonist complexes. At the intracellular surface, different conformations of helix VI observed for partial and full agonist complexes manifest a correlation between the efficacy-related structural rearrangement of this activation motif and intracellular signaling to partner proteins. While comparisons of A2AAR in complexes with partial and full agonists with different methods showed close similarity of the global folds, this NMR study now reveals subtle but distinct local structural differences related to partial agonism.
中文翻译:

A2A 腺苷受体部分激动与激活微动开关中的结构重排有关
在药物设计中,G 蛋白偶联受体 (GPCR) 部分激动剂使人们能够在基础和最大信号水平之间微调受体输出。在这里,我们增加了合理化和监测部分激动的结构基础。A 2A腺苷受体 (A 2A AR)的部分激动剂复合物的 NMR 波谱显示 PIF 激活基序的构象与完全激动剂复合物明显不同。在细胞内表面,部分和完全激动剂复合物观察到的螺旋 VI 的不同构象表明该激活基序的功效相关结构重排与细胞内信号传导至伙伴蛋白之间存在相关性。而 A 2A 的比较使用不同方法与部分和完全激动剂形成的复合物中的 AR 显示出整体折叠的密切相似性,这项 NMR 研究现在揭示了与部分激动相关的微妙但明显的局部结构差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号