Solid State Nuclear Magnetic Resonance ( IF 1.8 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.ssnmr.2020.101701 Joel Lapin 1 , Emmanuel O Awosanya 1 , Richard J A Esteves 1 , Alexander A Nevzorov 1
|
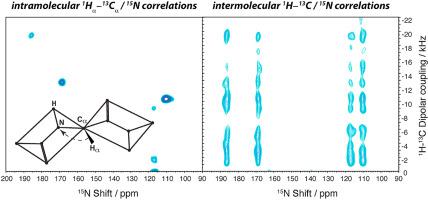
The benefits of triple-resonance experiments for structure determination of macroscopically oriented membrane proteins by solid-state NMR are discussed. While double-resonance 1H/15N experiments are effective for structure elucidation of alpha-helical domains, extension of the method of oriented samples to more complex topologies and assessing side-chain conformations necessitates further development of triple-resonance (1H/13C/15N) NMR pulse sequences. Incorporating additional spectroscopic dimensions involving 13C spin-bearing nuclei, however, introduces essential complications arising from the wide frequency range of the 1H-13C dipolar couplings and 13C CSA (>20 kHz), and the presence of the 13C-13C homonuclear dipole-dipole interactions. The recently reported ROULETTE–CAHA pulse sequence, in combination with the selective z-filtering, can be used to evolve the structurally informative 1H-13C dipolar coupling arising from the aliphatic carbons while suppressing the signals from the carbonyl and methyl regions. Proton-mediated magnetization transfer under mismatched Hartman-Hahn conditions (MMHH) can be used to correlate 13C and 15N nuclei in such triple-resonance experiments for the subsequent 15N detection. The recently developed pulse sequences are illustrated for n-acetyl Leucine (NAL) single crystal and doubly labeled Pf1 coat protein reconstituted in magnetically aligned bicelles. An interesting observation is that in the case of 15N-labeled NAL measured at 13C natural abundance, the triple (1H/13C/15N) MMHH scheme predominantly gives rise to long-range intermolecular magnetization transfers from 13C to 15N spins; whereas direct Hartmann-Hahn 13C/15N transfer is entirely intramolecular. The presented developments advance NMR of oriented samples for structure determination of membrane proteins and liquid crystals.
中文翻译:

定向样品核磁共振对膜蛋白结构测定的1H/13C/15N三重共振实验
讨论了三重共振实验对通过固态 NMR 确定宏观定向膜蛋白结构的好处。虽然双共振1 H/ 15 N 实验对于 α 螺旋结构域的结构解析是有效的,但将定向样品的方法扩展到更复杂的拓扑结构和评估侧链构象需要进一步发展三共振 ( 1 H/ 13 C/ 15 N) NMR 脉冲序列。然而,结合涉及13 C 自旋轴承核的额外光谱尺寸,引入了由1 H- 13的宽频率范围引起的基本复杂性C偶极耦合和13 C CSA (>20 kHz),以及13 C- 13 C同核偶极-偶极相互作用的存在。最近报道的 ROULETTE-CAHA 脉冲序列与选择性 z 滤波相结合,可用于演化由脂肪碳产生的结构信息丰富的1 H- 13 C 偶极耦合,同时抑制来自羰基和甲基区域的信号。在不匹配的Hartman -Hahn 条件 (MMHH) 下质子介导的磁化转移可用于在随后的15N 检测。最近开发的脉冲序列用于 n-乙酰亮氨酸 (NAL) 单晶和双重标记的 Pf1 外壳蛋白,并在磁性对齐的 bicelles 中重组。一个有趣的观察结果是,在15 N 标记的 NAL 在13 C 自然丰度下测量的情况下,三重 ( 1 H/ 13 C/ 15 N) MMHH 方案主要引起从13 C 到15的长程分子间磁化转移N 次旋转;而直接 Hartmann-Hahn 13 C/ 15N转移完全是分子内的。所提出的进展促进了定向样品的核磁共振,用于膜蛋白和液晶的结构测定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号