Journal of Advanced Research ( IF 11.4 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.jare.2020.11.009 Shu-Hui Wu, Chia-Chu Hsieh, Szu-Chun Hsu, Ming Yao, Jong-Kai Hsiao, Shih-Wei Wang, Chih-Peng Lin, Dong-Ming Huang
|
Introduction
Chemotherapeutic drugs are the main intervention for cancer management, but many drawbacks impede their clinical applications. Nanoparticles as drug delivery systems (DDSs) offer much promise to solve these limitations.
Objectives
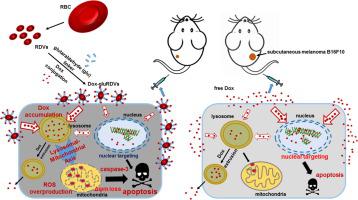
A novel nanocarrier composed of red blood cell (RBC)-derived vesicles (RDVs) surface-linked with doxorubicin (Dox) using glutaraldehyde (glu) to form Dox-gluRDVs was investigated for improved cancer therapy.
Methods
We investigated the in vivo antineoplastic performance of Dox-gluRDVs through intravenous (i.v.) administration in the mouse model bearing subcutaneous (s.c.) B16F10 tumor and examined the in vitro antitumor mechanism and efficacy in a panel of cancer cell lines.
Results
Dox-gluRDVs can exert superior anticancer activity than free Dox in vitro and in vivo. Distinct from free Dox that is mainly located in the nucleus, but instead Dox-gluRDVs release and efficiently deliver the majority of their conjugated Dox into lysosomes. In vitro mechanism study reveals the critical role of lysosomal Dox accumulation-mediated mitochondrial ROS overproduction followed by the mitochondrial membrane potential loss and the activation of apoptotic signaling for superior anticancer activity of Dox-gluRDVs.
Conclusion
This work demonstrates the great potential of RDVs to serve a biological DDS of Dox for systemic administration to improve conventional cancer chemotherapeutics.
中文翻译:

红细胞衍生的囊泡作为多柔比星的全身递送系统用于溶酶体 - 线粒体轴改善的癌症治疗
介绍
化疗药物是癌症管理的主要干预手段,但许多缺点阻碍了它们的临床应用。纳米颗粒作为药物递送系统 (DDS) 为解决这些限制提供了很大希望。
目标
研究了一种由红细胞 (RBC) 衍生的囊泡 (RDV) 与阿霉素 (Dox) 表面连接的新型纳米载体,使用戊二醛 (glu) 形成 Dox-gluRDV,以改善癌症治疗。
方法
我们通过静脉内 ( iv ) 给药研究了Dox-gluRDV在皮下 ( sc ) B16F10 肿瘤小鼠模型中的体内抗肿瘤性能,并检查了一组癌细胞系的体外抗肿瘤机制和功效。
结果
在体外和体内, Dox-gluRDVs 可以发挥比游离 Dox 更好的抗癌活性。与主要位于细胞核中的游离 Dox 不同,而是 Dox-gluRDV 释放并有效地将大部分结合的 Dox 递送到溶酶体中。体外机制研究揭示了溶酶体 Dox 积累介导的线粒体 ROS 过量产生的关键作用,随后是线粒体膜电位损失和凋亡信号传导的激活,以实现 Dox-gluRDV 的优异抗癌活性。
结论
这项工作证明了 RDV 的巨大潜力,可以作为 Dox 的生物 DDS 进行全身给药,以改善传统的癌症化疗药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号