当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The pathways and domain specificity of Cu(I) binding to human metallothionein 1A
Metallomics ( IF 2.9 ) Pub Date : 2020-11-6 , DOI: 10.1039/d0mt00215a Adyn Melenbacher 1 , Natalie C Korkola , Martin J Stillman
Metallomics ( IF 2.9 ) Pub Date : 2020-11-6 , DOI: 10.1039/d0mt00215a Adyn Melenbacher 1 , Natalie C Korkola , Martin J Stillman
Affiliation
|
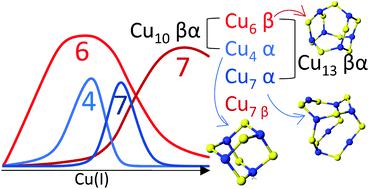
Copper is an essential element, but as a result of numerous adverse reactions, it is also a cellular toxin. Nature protects itself from these toxic reactions by binding cuprous copper to chaperones and other metalloproteins. Metallothionein has been proposed as a storage location for Cu(I) and potentially as the donor of Cu(I) to copper-dependent enzymes. We report that the addition of Cu(I) to apo recombinant human metallothionein 1a cooperatively forms a sequential series of Cu(I)–cysteinyl thiolate complexes that have specific Cu(I) : MT stoichiometries of 6 : 1, 10 : 1, and finally 13 : 1. The individual domain Cu : SCys stoichiometries were determined as Cu6S9 (for 6 : 1), Cu6S9 + Cu4S6 (for 10 : 1), and Cu6S9 + Cu7S9 (for 13 : 1) based on the number of modified free cysteines not involved in Cu(I) binding. The stoichiometries are associated with Cu–SCys cluster formation involving bridging thiols in the manner similar to the clusters formed with Cd(II) and Zn(II). The locations of these clustered species within the 20 cysteine full protein were determined from the unique speciation profiles of Cu(I) binding to the β and α domain fragments of recombinant human metallothionein 1a with 9 and 11 cysteines, respectively. Competition reactions using these domain fragments challenged Cu(I) metallation of the βα protein, allowing the sequence of cluster formation in the full protein to be determined. Relative binding constants for each Cu(I) bound are reported. The emission spectra of the Cu4S6, Cu6S9, and Cu7S9 clusters have unique λmax and phosphorescent lifetime properties. These phosphorescent data provide unambiguous supporting evidence for the presence of solvent shielded clusters reported concurrently by ESI-MS. Simulated emission spectra based on the cluster specific emission profiles matched the experimental spectra and are used to confirm that the relative concentrations seen by ESI-MS are representative of the solution. Our results suggest that the availability of a series of sequential Cu(I)–thiolate clusters provides flexibility as a means of protecting the cell from toxicity while still allowing for homeostatic control of the total copper content in the cell. This mechanism provides a dynamic and reactive method of reducing the cellular free copper concentrations.
中文翻译:

Cu(I) 与人金属硫蛋白 1A 结合的途径和域特异性
铜是一种必需元素,但由于许多不良反应,它也是一种细胞毒素。大自然通过将亚铜铜与分子伴侣和其他金属蛋白结合来保护自己免受这些毒性反应的影响。金属硫蛋白已被提议作为 Cu( I )的储存位置,并可能作为铜依赖酶的 Cu( I )供体。我们报告说,将 Cu( I ) 添加到 apo 重组人金属硫蛋白 1a 中协同形成了一系列连续的 Cu( I )-半胱氨酰硫醇盐络合物,这些络合物具有特定的 Cu( I ):MT 化学计量比为 6:1、10:1 和最后 13 : 1. 单个域 Cu : S Cys化学计量被确定为 Cu 6S 9 (对于 6 : 1)、Cu 6 S 9 + Cu 4 S 6 (对于 10 : 1) 和 Cu 6 S 9 + Cu 7 S 9 (对于 13 : 1) 基于未修饰的游离半胱氨酸的数量参与 Cu( I ) 结合。化学计量与 Cu-S Cys簇的形成有关,涉及桥接硫醇,其方式类似于用 Cd( II ) 和 Zn( II )形成的簇。这些簇状物种在 20 个半胱氨酸全蛋白中的位置是根据 Cu( I) 分别与具有 9 个和 11 个半胱氨酸的重组人金属硫蛋白 1a 的 β 和 α 结构域片段结合。使用这些结构域片段的竞争反应挑战了 βα 蛋白的Cu( I ) 金属化,从而可以确定完整蛋白质中簇形成的序列。报告了每个 Cu( I ) 结合的相对结合常数。Cu 4 S 6、Cu 6 S 9和Cu 7 S 9簇的发射光谱具有唯一的λ max和磷光寿命特性。这些磷光数据为 ESI-MS 同时报告的溶剂屏蔽簇的存在提供了明确的支持证据。基于簇特定发射剖面的模拟发射光谱与实验光谱相匹配,用于确认 ESI-MS 看到的相对浓度代表溶液。我们的结果表明,一系列连续的 Cu( I )-硫醇盐簇的可用性提供了灵活性,作为保护细胞免受毒性的一种手段,同时仍然允许对细胞中总铜含量进行稳态控制。这种机制提供了一种降低细胞游离铜浓度的动态和反应性方法。
更新日期:2021-01-06
中文翻译:

Cu(I) 与人金属硫蛋白 1A 结合的途径和域特异性
铜是一种必需元素,但由于许多不良反应,它也是一种细胞毒素。大自然通过将亚铜铜与分子伴侣和其他金属蛋白结合来保护自己免受这些毒性反应的影响。金属硫蛋白已被提议作为 Cu( I )的储存位置,并可能作为铜依赖酶的 Cu( I )供体。我们报告说,将 Cu( I ) 添加到 apo 重组人金属硫蛋白 1a 中协同形成了一系列连续的 Cu( I )-半胱氨酰硫醇盐络合物,这些络合物具有特定的 Cu( I ):MT 化学计量比为 6:1、10:1 和最后 13 : 1. 单个域 Cu : S Cys化学计量被确定为 Cu 6S 9 (对于 6 : 1)、Cu 6 S 9 + Cu 4 S 6 (对于 10 : 1) 和 Cu 6 S 9 + Cu 7 S 9 (对于 13 : 1) 基于未修饰的游离半胱氨酸的数量参与 Cu( I ) 结合。化学计量与 Cu-S Cys簇的形成有关,涉及桥接硫醇,其方式类似于用 Cd( II ) 和 Zn( II )形成的簇。这些簇状物种在 20 个半胱氨酸全蛋白中的位置是根据 Cu( I) 分别与具有 9 个和 11 个半胱氨酸的重组人金属硫蛋白 1a 的 β 和 α 结构域片段结合。使用这些结构域片段的竞争反应挑战了 βα 蛋白的Cu( I ) 金属化,从而可以确定完整蛋白质中簇形成的序列。报告了每个 Cu( I ) 结合的相对结合常数。Cu 4 S 6、Cu 6 S 9和Cu 7 S 9簇的发射光谱具有唯一的λ max和磷光寿命特性。这些磷光数据为 ESI-MS 同时报告的溶剂屏蔽簇的存在提供了明确的支持证据。基于簇特定发射剖面的模拟发射光谱与实验光谱相匹配,用于确认 ESI-MS 看到的相对浓度代表溶液。我们的结果表明,一系列连续的 Cu( I )-硫醇盐簇的可用性提供了灵活性,作为保护细胞免受毒性的一种手段,同时仍然允许对细胞中总铜含量进行稳态控制。这种机制提供了一种降低细胞游离铜浓度的动态和反应性方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号