当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Temperature Effects on CO2 Electroreduction Pathways in an Imidazolium-Based Ionic Liquid on Pt Electrode
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jpcc.0c06065 Tianrong Zhan 1, 2 , Anil Kumar 1 , Michael Sevilla 1 , Arun Sridhar 1 , Xiangqun Zeng 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jpcc.0c06065 Tianrong Zhan 1, 2 , Anil Kumar 1 , Michael Sevilla 1 , Arun Sridhar 1 , Xiangqun Zeng 1
Affiliation
|
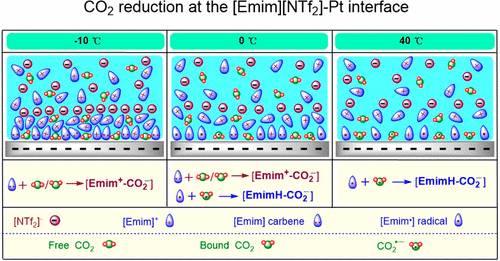
Ionic liquids (ILs) are thermally stable at both high and low temperatures, making them excellent solvents and electrolytes in extreme ambient conditions. In this work, we systematically studied the temperature effects on the various pathways of electrochemical reduction of CO2 in 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Emim][NTf2]) and compared them with those in 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ([Bmpy][NTf2]) by electrochemical methods and density functional calculations. We summarize the crucial factors determining the reaction pathways for electroreduction of CO2 in [Emim][NTf2]. Our results show that temperature can be used to modulate the reaction pathway of electrochemical reduction of carbon dioxide in this imidazolium-based IL via the effects on (i) the mass transport of the reactant and intermediates, (ii) the adsorption and solubility of the intermediates species, and (iii) the IL–electrode interface structure and properties.
中文翻译:

Pt电极上咪唑基离子液体对CO 2电还原途径的温度影响
离子液体(IL)在高温和低温下均具有热稳定性,使其在极端环境条件下成为出色的溶剂和电解质。在这项工作中,我们系统地研究了温度对1-乙基-3-甲基咪唑双(三氟甲基磺酰基)酰亚胺([Emim] [NTf 2 ])中CO 2电化学还原的各种途径的影响,并将它们与1-丁基-1-甲基吡咯烷鎓双(三氟甲基磺酰基)酰亚胺([Bmpy] [NTf 2 ])的电化学方法和密度泛函计算。我们总结了决定[Emim] [NTf 2 ]中CO 2电还原反应途径的关键因素]。我们的结果表明,温度可通过以下方式调节该咪唑基IL中二氧化碳的电化学还原反应途径:(i)反应物和中间体的传质,(ii)吸附和溶解度。中间体种类,以及(iii)IL-电极界面的结构和性质。
更新日期:2020-12-03
中文翻译:

Pt电极上咪唑基离子液体对CO 2电还原途径的温度影响
离子液体(IL)在高温和低温下均具有热稳定性,使其在极端环境条件下成为出色的溶剂和电解质。在这项工作中,我们系统地研究了温度对1-乙基-3-甲基咪唑双(三氟甲基磺酰基)酰亚胺([Emim] [NTf 2 ])中CO 2电化学还原的各种途径的影响,并将它们与1-丁基-1-甲基吡咯烷鎓双(三氟甲基磺酰基)酰亚胺([Bmpy] [NTf 2 ])的电化学方法和密度泛函计算。我们总结了决定[Emim] [NTf 2 ]中CO 2电还原反应途径的关键因素]。我们的结果表明,温度可通过以下方式调节该咪唑基IL中二氧化碳的电化学还原反应途径:(i)反应物和中间体的传质,(ii)吸附和溶解度。中间体种类,以及(iii)IL-电极界面的结构和性质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号