当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Mechanism of the Dehydration of Calcium Sulfate Dihydrate: A Comprehensive Approach for Studying the Dehydration of Ionic Hydrates under Controlled Temperature and Water Vapor Pressure
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jpcc.0c09041 João G. D. Preturlan 1, 2 , Laetitia Vieille 1 , Sara Quiligotti 2 , Loïc Favergeon 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jpcc.0c09041 João G. D. Preturlan 1, 2 , Laetitia Vieille 1 , Sara Quiligotti 2 , Loïc Favergeon 1
Affiliation
|
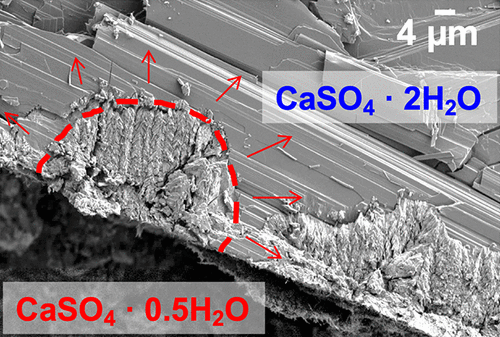
We studied the kinetics and mechanism of the dehydration reaction of calcium sulfate dihydrate to hemihydrate under controlled temperature and water vapor partial pressure. From kinetic and reaction rate curves obtained using thermogravimetric analysis (TGA) under isothermal and isobaric conditions, we determined the overall behavior of this dehydration reaction and the effects of the system’s intensive variables on its kinetics. We observed that the reactions take place with an initial induction period that decreases with increasing temperature, followed by a sigmoidal mass loss controlled by both nucleation and growth processes. Characterization of our samples at different points of the reaction allowed us to observe and confirm a surface nucleation process followed by isotropic growth of the nuclei with inward development of the solid product. We then employed the Mampel kinetic model based on the observed experimental results considering the physical nature of the investigated transformation and the real geometry of the particles. From this model, we obtained sets of kinetic parameters for the nucleation and growth processes and their evolution with temperature. We then proposed physicochemical mechanisms for both processes, and they were considered to interpret the kinetic parameters obtained previously. The mechanistic analysis of the system allowed determination of the effects of both temperature and water vapor pressure on the kinetic behavior of the reaction, which corresponds to a novel approach for the dehydration reaction of calcium sulfate dihydrate. The universal kinetic approach used to treat this chemical system in this work can be applied for studying the dehydration of other ionic hydrates.
中文翻译:

二水合硫酸钙脱水的动力学和机理:控制温度和水蒸气压下离子水合物脱水的综合研究方法
我们研究了在控制温度和水蒸气分压下,二水合硫酸钙脱水成半水合物的动力学和机理。根据在等温和等压条件下使用热重分析(TGA)获得的动力学和反应速率曲线,我们确定了该脱水反应的总体行为以及系统强度变量对其动力学的影响。我们观察到,反应发生的起始诱导期随温度升高而降低,随后是由成核和生长过程控制的S形质量损失。在反应的不同点对样品进行表征,使我们能够观察并确认表面成核过程,随后是核的各向同性生长以及固体产物向内发展。然后,基于观察到的实验结果,我们考虑了所研究的转变的物理性质和粒子的实际几何形状,采用了Mampel动力学模型。从该模型中,我们获得了成核和生长过程及其随温度变化的动力学参数集。然后,我们为这两个过程提出了理化机理,并认为它们可以解释先前获得的动力学参数。系统的机械分析可以确定温度和水蒸气压对反应动力学行为的影响,这对应于二水合硫酸钙脱水反应的新方法。
更新日期:2020-12-03
中文翻译:

二水合硫酸钙脱水的动力学和机理:控制温度和水蒸气压下离子水合物脱水的综合研究方法
我们研究了在控制温度和水蒸气分压下,二水合硫酸钙脱水成半水合物的动力学和机理。根据在等温和等压条件下使用热重分析(TGA)获得的动力学和反应速率曲线,我们确定了该脱水反应的总体行为以及系统强度变量对其动力学的影响。我们观察到,反应发生的起始诱导期随温度升高而降低,随后是由成核和生长过程控制的S形质量损失。在反应的不同点对样品进行表征,使我们能够观察并确认表面成核过程,随后是核的各向同性生长以及固体产物向内发展。然后,基于观察到的实验结果,我们考虑了所研究的转变的物理性质和粒子的实际几何形状,采用了Mampel动力学模型。从该模型中,我们获得了成核和生长过程及其随温度变化的动力学参数集。然后,我们为这两个过程提出了理化机理,并认为它们可以解释先前获得的动力学参数。系统的机械分析可以确定温度和水蒸气压对反应动力学行为的影响,这对应于二水合硫酸钙脱水反应的新方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号