当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibria of Mn2+,NH4+//SO42–,Cl––H2O and Its Subsystems (Mn2+,NH4+//Cl––H2O, Mn2+//SO42–,Cl––H2O) at T = 303.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jced.0c00155 Yujia Zhang 1 , Bingjie Yu 1 , Yongsheng Ren 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jced.0c00155 Yujia Zhang 1 , Bingjie Yu 1 , Yongsheng Ren 1
Affiliation
|
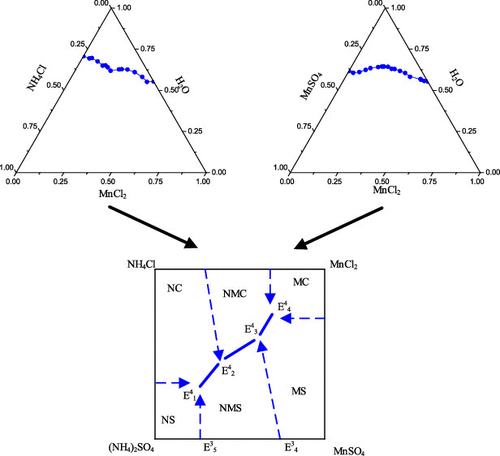
In this study, the solid–liquid equilibrium data of the quaternary system Mn2+,NH4+//SO42–,Cl––H2O and its two ternary subsystems (Mn2+,NH4+//Cl––H2O and Mn2+//SO42–,Cl––H2O) were measured by the isothermal dissolution method. For the two ternary systems, the solubility of the equilibrium liquid phase was determined. In the Mn2+,NH4+//Cl––H2O system, there are two invariant points and five crystallization regions. In the Mn2+//SO42–,Cl––H2O system, there are one invariant point and three crystallization regions. The solubility data of the quaternary system Mn2+,NH4+//SO42–,Cl––H2O were measured in the experiment. According to the theory of translation, the number of invariant points and crystallization zones of the quaternary system are predicted. The predicted results are the same as the experimental results, both of them have the same number of co-saturation points and crystallization zones. There are four invariant points and six crystallization regions in the quaternary system. It provides a theoretical basis for the water–salt system containing the quaternary system.
中文翻译:

Mn 2 +,NH 4 + // SO 4 2–,Cl – –H 2 O及其子系统的固液平衡(Mn 2 +,NH 4 + // Cl – –H 2 O,Mn 2+ / / SO 4 2–,Cl – –H 2 O)在T = 303.15 K
在这项研究中,四元系统Mn 2 +,NH 4 + // SO 4 2–,Cl – H 2 O及其两个三元子系统(Mn 2 +,NH 4 + // Cl - -H 2 O和锰2+ // SO 4 2-,氯- -H 2 O),通过等温溶解法测定的。对于两个三元体系,确定了平衡液相的溶解度。在Mn 2+中,NH 4 + // Cl – –H 2在系统中,有两个不变点和五个结晶区域。在Mn 2+ // SO 4 2–,Cl – –H 2 O系统中,存在一个不变点和三个结晶区。四元体系Mn 2 +,NH 4 + // SO 4 2–,Cl – –H 2的溶解度数据在实验中测量了O。根据平移理论,预测了四元体系的不变点数和结晶区。预测结果与实验结果相同,二者具有相同的共饱和点数和结晶区。四元体系中有四个不变点和六个结晶区。它为包含四元体系的水盐体系提供了理论基础。
更新日期:2021-01-14
中文翻译:

Mn 2 +,NH 4 + // SO 4 2–,Cl – –H 2 O及其子系统的固液平衡(Mn 2 +,NH 4 + // Cl – –H 2 O,Mn 2+ / / SO 4 2–,Cl – –H 2 O)在T = 303.15 K
在这项研究中,四元系统Mn 2 +,NH 4 + // SO 4 2–,Cl – H 2 O及其两个三元子系统(Mn 2 +,NH 4 + // Cl - -H 2 O和锰2+ // SO 4 2-,氯- -H 2 O),通过等温溶解法测定的。对于两个三元体系,确定了平衡液相的溶解度。在Mn 2+中,NH 4 + // Cl – –H 2在系统中,有两个不变点和五个结晶区域。在Mn 2+ // SO 4 2–,Cl – –H 2 O系统中,存在一个不变点和三个结晶区。四元体系Mn 2 +,NH 4 + // SO 4 2–,Cl – –H 2的溶解度数据在实验中测量了O。根据平移理论,预测了四元体系的不变点数和结晶区。预测结果与实验结果相同,二者具有相同的共饱和点数和结晶区。四元体系中有四个不变点和六个结晶区。它为包含四元体系的水盐体系提供了理论基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号