当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Research on the 2-Chloro-4-amino-6,7-dimethoxyquinazoline Solubility in 12 Monosolvents at Various Temperatures: Experimental Measurement and Thermodynamic Correlation
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jced.0c00506 Guoquan Zhou 1 , Ke Chen 2 , Zehui Yang 1 , Danfeng Shao 1 , Huajun Fan 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.jced.0c00506 Guoquan Zhou 1 , Ke Chen 2 , Zehui Yang 1 , Danfeng Shao 1 , Huajun Fan 3
Affiliation
|
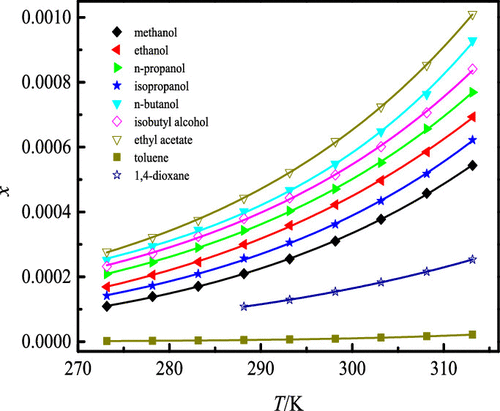
The present study investigating the experimental equilibrium solubility of 2-chloro-4-amino-6,7-dimethoxyquinazoline (CADQ) dissolved in 12 kinds of neat organic solvents, including methanol, ethanol, n-propanol, isopropanol, n-butanol, isobutyl alcohol, ethyl acetate, toluene, N,N-dimethyl formamide (DMF), N-methylpyrrolidone (NMP), 1,4-dioxane, and cyclohexanone, was determined in an equilibrium state by means of the isothermal saturation method at temperature ranges of 273.15–313.15 K under normal atmospheric conditions of 101.2 kPa. The rising temperature showed the obvious positive effect on the increasing CADQ solubility gradually, and the maximum value (0.01370 at 313.15 K, in mole fraction) was observed in NMP for the studied temperature ranges. The equilibrated solid state of CADQ was characterized by applying the Fourier transform infrared (FT-IR) spectrometer, resulting in no existences of crystal transition, solvate formation, or polymorphic transformation in the experimental process. Results showed that the mole fraction solubility of CADQ abiding the order from high to low is as follows: NMP (0.01370, 313.15 K) > DMF (0.01157, 313.15 K) > cyclohexanone (3.847 × 10–3, 313.15 K) > ethyl acetate (1.010 × 10–3, 313.15 K) > n-butanol (9.288 × 10–4, 313.15 K) > isobutyl alcohol (8.408 × 10–4, 313.15 K) > n-propanol (7.692 × 10–4, 313.15 K) > ethanol (6.934 × 10–4, 313.15 K) > isopropanol (6.220 × 10–4, 313.15 K) > methanol (5.437 × 10–4, 313.15 K) > 1,4-dioxane (2.535 × 10–4, 313.15 K) > toluene (2.174 × 10–5, 313.15 K). Then, the achieved CADQ solubility values were correlated and calculated by mathematical models containing the modified Apelblat equation and λh equation, respectively. As a consequence, the largest root-mean-square deviation (RMSD) and average relative deviation (ARD) were 1.020 × 10–4 and 2.352%, respectively, which turned out to be that the calculated data agreed well with the experimental ones.
中文翻译:

不同温度下12种单溶剂中2-氯-4-氨基-6,7-二甲氧基喹唑啉溶解度的研究:实验测量和热力学相关性
本研究调查了2-氯-4-氨基-6,7-二甲氧基喹唑啉(CADQ)溶于12种纯有机溶剂(包括甲醇,乙醇,正丙醇,异丙醇,正丁醇,异丁基)的实验平衡溶解度醇,乙酸乙酯,甲苯,N,N-二甲基甲酰胺(DMF),N甲基吡咯烷酮(NMP),1,4-二恶烷和环己酮是通过等温饱和法在273.15–313.15 K的温度范围内,101.2 kPa的正常大气条件下以平衡状态测定的。升高的温度对逐渐增加CADQ的溶解度显示出明显的积极作用,在所研究的温度范围内,NMP的最大值(摩尔分数为313.15 K时为0.01370)达到最大值。通过使用傅立叶变换红外(FT-IR)光谱仪对CADQ的平衡固态进行表征,从而在实验过程中不存在晶体转变,溶剂化物形成或多晶型转变。结果表明,CADQ的摩尔分数溶解度从高到低依次为:NMP(0.01370,313.15 K)> DMF(0.01157,313)。-3,313.15 K)>乙酸乙酯(1.010×10 -3,313.15 K)> Ñ丁醇(9.288×10 -4,313.15 K)>异丁醇(8.408×10 -4,313.15 K)> Ñ丙醇(7.692×10 -4,313.15 K)>乙醇(6.934×10 -4,313.15 K)>异丙醇(6.220×10 -4,313.15 K)>甲醇(5.437×10 -4,313.15 K)> 1,4-二恶烷(2.535×10 -4,313.15 K)>甲苯(2.174×10 -5,313.15 K)。然后,将取得CADQ溶解度值是相关的,并通过含有改性Apelblat方程和λ的数学模型计算出ħ等式。结果,最大均方根偏差(RMSD)和平均相对偏差(ARD)分别为1.020×10 –4和2.352%,这表明计算数据与实验数据吻合良好。
更新日期:2021-01-14
中文翻译:

不同温度下12种单溶剂中2-氯-4-氨基-6,7-二甲氧基喹唑啉溶解度的研究:实验测量和热力学相关性
本研究调查了2-氯-4-氨基-6,7-二甲氧基喹唑啉(CADQ)溶于12种纯有机溶剂(包括甲醇,乙醇,正丙醇,异丙醇,正丁醇,异丁基)的实验平衡溶解度醇,乙酸乙酯,甲苯,N,N-二甲基甲酰胺(DMF),N甲基吡咯烷酮(NMP),1,4-二恶烷和环己酮是通过等温饱和法在273.15–313.15 K的温度范围内,101.2 kPa的正常大气条件下以平衡状态测定的。升高的温度对逐渐增加CADQ的溶解度显示出明显的积极作用,在所研究的温度范围内,NMP的最大值(摩尔分数为313.15 K时为0.01370)达到最大值。通过使用傅立叶变换红外(FT-IR)光谱仪对CADQ的平衡固态进行表征,从而在实验过程中不存在晶体转变,溶剂化物形成或多晶型转变。结果表明,CADQ的摩尔分数溶解度从高到低依次为:NMP(0.01370,313.15 K)> DMF(0.01157,313)。-3,313.15 K)>乙酸乙酯(1.010×10 -3,313.15 K)> Ñ丁醇(9.288×10 -4,313.15 K)>异丁醇(8.408×10 -4,313.15 K)> Ñ丙醇(7.692×10 -4,313.15 K)>乙醇(6.934×10 -4,313.15 K)>异丙醇(6.220×10 -4,313.15 K)>甲醇(5.437×10 -4,313.15 K)> 1,4-二恶烷(2.535×10 -4,313.15 K)>甲苯(2.174×10 -5,313.15 K)。然后,将取得CADQ溶解度值是相关的,并通过含有改性Apelblat方程和λ的数学模型计算出ħ等式。结果,最大均方根偏差(RMSD)和平均相对偏差(ARD)分别为1.020×10 –4和2.352%,这表明计算数据与实验数据吻合良好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号