当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Origin of Stereoselectivity in FLP-Catalyzed Asymmetric Hydrogenation of Imines
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-23 , DOI: 10.1021/acscatal.0c04263 Andrea Hamza 1 , Kristina Sorochkina 2 , Bianka Kótai 1 , Konstantin Chernichenko 2 , Dénes Berta 1 , Michael Bolte 3 , Martin Nieger 2 , Timo Repo 2 , Imre Pápai 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-11-23 , DOI: 10.1021/acscatal.0c04263 Andrea Hamza 1 , Kristina Sorochkina 2 , Bianka Kótai 1 , Konstantin Chernichenko 2 , Dénes Berta 1 , Michael Bolte 3 , Martin Nieger 2 , Timo Repo 2 , Imre Pápai 1
Affiliation
|
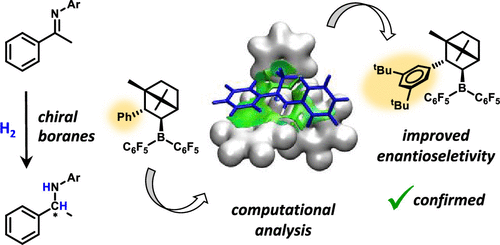
Development of metal-free strategies for stereoselective hydrogenation of unsaturated substrates is of particular interest in asymmetric synthesis. The emerging chemistry of frustrated Lewis pairs offers a promising approach along this line as demonstrated by recent achievements. However, the stereocontrol elements in these reactions are not clearly recognized thus far. Herein, we analyze the origin of stereoinduction in direct hydrogenation of imines catalyzed by a set of chiral boranes. We use the tools of computational chemistry to describe the elementary steps of the catalytic cycle, and we pay special attention to the stereoselectivity-determining hydride transfer process. The enantioselectivities predicted by the applied computational approach are in very good agreement with previous experimental observations. We find that the stereoselectivity is governed by a thermodynamically less favored conformer of the borohydride intermediate and not by the experimentally observed form. The most favored hydride transfer transition states are stabilized by specific aryl–aryl and alkyl–aryl noncovalent interactions, which play an important role in stereoinduction. This computational insight is exploited in proposing additional borane variants to improve the enantioselectivity, which could be demonstrated experimentally
中文翻译:

FLP催化的亚胺不对称氢化中立体选择性的起源
在不对称合成中,特别需要开发用于不饱和底物的立体选择性氢化的无金属策略。沮丧的刘易斯对的新兴化学方法提供了一种有前途的方法,如最近的成就所证明。但是,到目前为止,尚不清楚这些反应中的立体控制元件。本文中,我们分析了由一组手性硼烷催化的亚胺直接加氢中立体诱导的起源。我们使用计算化学的工具来描述催化循环的基本步骤,并特别注意确定氢化物转移的立体选择性。通过应用计算方法预测的对映选择性与先前的实验观察非常吻合。我们发现,立体选择性是由硼氢化物中间体的热力学上较不受欢迎的构象异构体决定的,而不是由实验观察到的形式决定的。最有利的氢化物转移过渡态通过特定的芳基-芳基和烷基-芳基非共价相互作用而稳定,这在立体诱导中起重要作用。这种计算洞察力被用于提出其他硼烷变体以提高对映选择性的实验中可以证明这一点。
更新日期:2020-12-04
中文翻译:

FLP催化的亚胺不对称氢化中立体选择性的起源
在不对称合成中,特别需要开发用于不饱和底物的立体选择性氢化的无金属策略。沮丧的刘易斯对的新兴化学方法提供了一种有前途的方法,如最近的成就所证明。但是,到目前为止,尚不清楚这些反应中的立体控制元件。本文中,我们分析了由一组手性硼烷催化的亚胺直接加氢中立体诱导的起源。我们使用计算化学的工具来描述催化循环的基本步骤,并特别注意确定氢化物转移的立体选择性。通过应用计算方法预测的对映选择性与先前的实验观察非常吻合。我们发现,立体选择性是由硼氢化物中间体的热力学上较不受欢迎的构象异构体决定的,而不是由实验观察到的形式决定的。最有利的氢化物转移过渡态通过特定的芳基-芳基和烷基-芳基非共价相互作用而稳定,这在立体诱导中起重要作用。这种计算洞察力被用于提出其他硼烷变体以提高对映选择性的实验中可以证明这一点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号