当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N,N‐Dimethoxyimidazolium Derivatives as Ion Pair Constituents of Energetic Redox Couples: Model Studies by Thermal Analysis and Crystallography
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2020-11-22 , DOI: 10.1002/zaac.202000364 Lukas Fliri 1, 2 , Thomas Gelbrich 3 , Ulrich J. Griesser 3 , Gabriel Partl 4 , Felix R. S. Purtscher 1 , Sandro Neuner 1 , Kevin Erharter 1 , Klaus Wurst 1 , Volker Kahlenberg 5 , Doris E. Braun 3 , Thomas S. Hofer 1 , Albert Rössler 4 , Herwig Schottenberger 1
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.1 ) Pub Date : 2020-11-22 , DOI: 10.1002/zaac.202000364 Lukas Fliri 1, 2 , Thomas Gelbrich 3 , Ulrich J. Griesser 3 , Gabriel Partl 4 , Felix R. S. Purtscher 1 , Sandro Neuner 1 , Kevin Erharter 1 , Klaus Wurst 1 , Volker Kahlenberg 5 , Doris E. Braun 3 , Thomas S. Hofer 1 , Albert Rössler 4 , Herwig Schottenberger 1
Affiliation
|
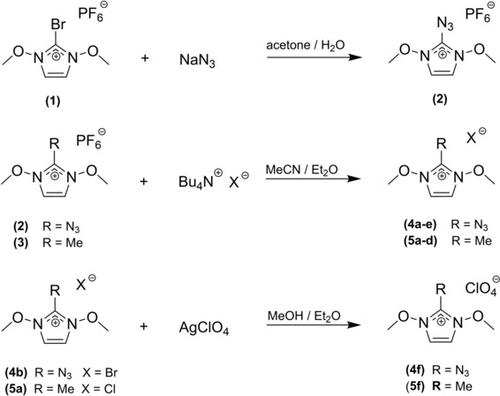
By utilizing an expedient anion exchange protocol and starting with an improved synthesis of 2‐azido‐1,3‐dimethoxyimidazolium hexafluorophosphate, a series of six metathetical salts, including the energetic nitrate and perchlorate as well as the halides, was prepared. We focused on the crystallographic and thermal characterization of these compounds, representing intrinsically reactive hybrid organic / inorganic salts, and conducted a small comparative study with the related 2‐methyl‐1,3‐dimethoxyimidazolium salts. The latter compounds were expected to thermally disintegrate less sluggishly since the covalent azido‐group as intrinsic blasting initiator is missing. Both 2‐azido‐ and 2‐methylimidazolium nitrate exhibit a very sharp thermal onset of complete disintegration. In contrast, the respective perchlorates display a continuous thermogravimetric weight loss over a broad temperature range. Additionally, X‐ray single crystal structure determinations were performed for eight of the new compounds as well as for two 2‐azido‐1,3‐dimethoxyimidazolium salts with a mixed anion composition. Phase purity was also checked for five of the congeners using powder X‐ray diffraction (Pawley fitting). Notably, in the 2‐azido‐imidazolium cation, the intra‐ring bonds of the N1−C2 type were found to be significantly shorter than N1−C4 type bonds, and a characteristic asymmetry between the two N1−C2−N(azido) bond angles was observed. The experimental cation geometry was reproduced by DFT calculations on selected compounds.
中文翻译:

N,N-二甲氧基咪唑鎓衍生物作为高能氧化还原对的离子对成分:通过热分析和晶体学进行的模型研究
通过使用方便的阴离子交换方案,并从改进的2-叠氮基-1,3-二甲氧基咪唑六氟磷酸酯的合成开始,制备了六种复分解盐,包括高能硝酸盐和高氯酸盐以及卤化物。我们专注于代表固有反应性杂化有机/无机盐的这些化合物的晶体学和热表征,并与相关的2-甲基-1,3-二甲氧基咪唑鎓盐进行了小型对比研究。由于缺少作为内在爆炸引发剂的共价叠氮基团,预计后一种化合物的热分解速度较慢。硝酸2-叠氮基和2-甲基咪唑鎓均表现出非常剧烈的完全崩解的热发作。相比之下,各自的高氯酸盐在较宽的温度范围内均显示出连续的热重失重。此外,还对8种新化合物以及2种带有混合阴离子组成的2-叠氮基-1,3-二甲氧基咪唑鎓盐进行了X射线单晶结构测定。还使用粉末X射线衍射(Pawley拟合)检查了五个同类物的相纯度。值得注意的是,在2-叠氮基咪唑阳离子中,N的环内键发现1 -C 2型明显小于N 1 -C 4型键,并且观察到两个N 1 -C 2 -N(叠氮基)键角之间的特征不对称性。通过对所选化合物进行DFT计算,再现了实验阳离子的几何形状。
更新日期:2020-11-22
中文翻译:

N,N-二甲氧基咪唑鎓衍生物作为高能氧化还原对的离子对成分:通过热分析和晶体学进行的模型研究
通过使用方便的阴离子交换方案,并从改进的2-叠氮基-1,3-二甲氧基咪唑六氟磷酸酯的合成开始,制备了六种复分解盐,包括高能硝酸盐和高氯酸盐以及卤化物。我们专注于代表固有反应性杂化有机/无机盐的这些化合物的晶体学和热表征,并与相关的2-甲基-1,3-二甲氧基咪唑鎓盐进行了小型对比研究。由于缺少作为内在爆炸引发剂的共价叠氮基团,预计后一种化合物的热分解速度较慢。硝酸2-叠氮基和2-甲基咪唑鎓均表现出非常剧烈的完全崩解的热发作。相比之下,各自的高氯酸盐在较宽的温度范围内均显示出连续的热重失重。此外,还对8种新化合物以及2种带有混合阴离子组成的2-叠氮基-1,3-二甲氧基咪唑鎓盐进行了X射线单晶结构测定。还使用粉末X射线衍射(Pawley拟合)检查了五个同类物的相纯度。值得注意的是,在2-叠氮基咪唑阳离子中,N的环内键发现1 -C 2型明显小于N 1 -C 4型键,并且观察到两个N 1 -C 2 -N(叠氮基)键角之间的特征不对称性。通过对所选化合物进行DFT计算,再现了实验阳离子的几何形状。











































 京公网安备 11010802027423号
京公网安备 11010802027423号