Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A highly conserved glutamic acid in ALFY inhibits membrane binding to aid in aggregate clearance
Traffic ( IF 3.6 ) Pub Date : 2020-11-22 , DOI: 10.1111/tra.12771 Erin F Reinhart 1 , Nicole A Litt 2 , Sarah Katzenell 1 , Maria Pellegrini 1 , Ai Yamamoto 2 , Michael J Ragusa 1, 3
Traffic ( IF 3.6 ) Pub Date : 2020-11-22 , DOI: 10.1111/tra.12771 Erin F Reinhart 1 , Nicole A Litt 2 , Sarah Katzenell 1 , Maria Pellegrini 1 , Ai Yamamoto 2 , Michael J Ragusa 1, 3
Affiliation
|
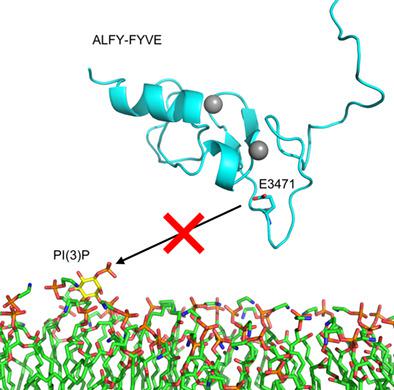
Autophagy‐linked FYVE protein (ALFY) is a large, multidomain protein involved in the degradation of protein aggregates by selective autophagy. The C‐terminal FYVE domain of ALFY has been shown to bind phosphatidylinositol 3‐phosphate (PI(3)P); however, ALFY only partially colocalizes with other FYVE domains in cells. Thus, we asked if the FYVE domain of ALFY has distinct membrane binding properties compared to other FYVE domains and whether these properties might affect its function in vivo. We found that the FYVE domain of ALFY binds weakly to PI(3)P containing membranes in vitro. This weak binding is the result of a highly conserved glutamic acid within the membrane insertion loop in the FYVE domain of ALFY that is not present in any other human FYVE domain. In addition, not only does this glutamic acid reduce binding to membranes in vitro and inhibits its targeting to membranes in vivo, but it is also important for the ability of ALFY to clear protein aggregates.
中文翻译:

ALFY 中高度保守的谷氨酸抑制膜结合,有助于聚集体清除
自噬相关 FYVE 蛋白 (ALFY) 是一种大型多结构域蛋白,参与选择性自噬降解蛋白质聚集体。ALFY 的 C 端 FYVE 结构域已被证明可结合磷脂酰肌醇 3-磷酸 (PI(3)P);然而,ALFY 仅部分与细胞中的其他 FYVE 结构域共定位。因此,我们询问 ALFY 的 FYVE 结构域与其他 FYVE 结构域相比是否具有独特的膜结合特性,以及这些特性是否可能影响其体内功能。我们发现 ALFY 的 FYVE 结构域在体外与含有 PI(3)P 的膜的结合较弱。这种弱结合是 ALFY 的 FYVE 结构域膜插入环内高度保守的谷氨酸的结果,而这种谷氨酸在任何其他人类 FYVE 结构域中都不存在。此外,这种谷氨酸不仅在体外减少与膜的结合并在体内抑制其对膜的靶向,而且对于ALFY清除蛋白质聚集体的能力也很重要。
更新日期:2021-01-29
中文翻译:

ALFY 中高度保守的谷氨酸抑制膜结合,有助于聚集体清除
自噬相关 FYVE 蛋白 (ALFY) 是一种大型多结构域蛋白,参与选择性自噬降解蛋白质聚集体。ALFY 的 C 端 FYVE 结构域已被证明可结合磷脂酰肌醇 3-磷酸 (PI(3)P);然而,ALFY 仅部分与细胞中的其他 FYVE 结构域共定位。因此,我们询问 ALFY 的 FYVE 结构域与其他 FYVE 结构域相比是否具有独特的膜结合特性,以及这些特性是否可能影响其体内功能。我们发现 ALFY 的 FYVE 结构域在体外与含有 PI(3)P 的膜的结合较弱。这种弱结合是 ALFY 的 FYVE 结构域膜插入环内高度保守的谷氨酸的结果,而这种谷氨酸在任何其他人类 FYVE 结构域中都不存在。此外,这种谷氨酸不仅在体外减少与膜的结合并在体内抑制其对膜的靶向,而且对于ALFY清除蛋白质聚集体的能力也很重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号