当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyridinic Nanographenes by Novel Precursor Design
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-11-23 , DOI: 10.1002/chem.202004983 David Reger 1 , Kilian Schöll 1 , Frank Hampel 1 , Harald Maid 1 , Norbert Jux 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-11-23 , DOI: 10.1002/chem.202004983 David Reger 1 , Kilian Schöll 1 , Frank Hampel 1 , Harald Maid 1 , Norbert Jux 1
Affiliation
|
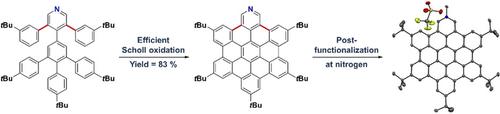
In this work we present the solution‐synthesis of pyridine analogues to hexa‐peri‐hexabenzocoronene (HBC)—which might be called superpyridines—via a novel precursor design. The key step in our strategy was the pre‐formation of the C−C bonds between the 3/3’ positions of the pyridine and the adjacent phenyl rings—bonds that are otherwise unreactive and difficult to close under Scholl‐conditions. Apart from the synthesis of the parent compound we show that classical pyridine chemistry, namely oxidation, N‐alkylation and metal‐coordination is applicable to the π‐extended analogue. Furthermore, we present basic physical chemical characterizations of the newly synthesized molecules. With this novel synthetic strategy, we hope to unlock the pyridine chemistry of nanographenes.
中文翻译:

新型前驱体设计的吡啶类纳米石墨烯
在这项工作中,我们提出的吡啶类似物的溶液合成来六围-hexabenzocoronene(HBC)哪位可能被称为superpyridines-通过新的前体的设计。我们策略中的关键步骤是在吡啶的3/3'位与相邻的苯环之间预先建立C-C键,否则这些键在Scholl条件下是无反应性且难以闭合的。除了母体化合物的合成外,我们还表明经典的吡啶化学,即氧化,N烷基化和金属配位可用于π扩展类似物。此外,我们介绍了新合成的分子的基本物理化学特征。通过这种新颖的合成策略,我们希望解锁纳米石墨烯的吡啶化学。
更新日期:2021-01-27
中文翻译:

新型前驱体设计的吡啶类纳米石墨烯
在这项工作中,我们提出的吡啶类似物的溶液合成来六围-hexabenzocoronene(HBC)哪位可能被称为superpyridines-通过新的前体的设计。我们策略中的关键步骤是在吡啶的3/3'位与相邻的苯环之间预先建立C-C键,否则这些键在Scholl条件下是无反应性且难以闭合的。除了母体化合物的合成外,我们还表明经典的吡啶化学,即氧化,N烷基化和金属配位可用于π扩展类似物。此外,我们介绍了新合成的分子的基本物理化学特征。通过这种新颖的合成策略,我们希望解锁纳米石墨烯的吡啶化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号