当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stable N,N’‐Diarylated Dihydrodiazaacene Radical Cations
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-12-30 , DOI: 10.1002/chem.202004548 Gaozhan Xie 1 , N. Maximilian Bojanowski 1 , Victor Brosius 1 , Thomas Wiesner 1 , Frank Rominger 1 , Jan Freudenberg 1 , Uwe H. F. Bunz 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-12-30 , DOI: 10.1002/chem.202004548 Gaozhan Xie 1 , N. Maximilian Bojanowski 1 , Victor Brosius 1 , Thomas Wiesner 1 , Frank Rominger 1 , Jan Freudenberg 1 , Uwe H. F. Bunz 1
Affiliation
|
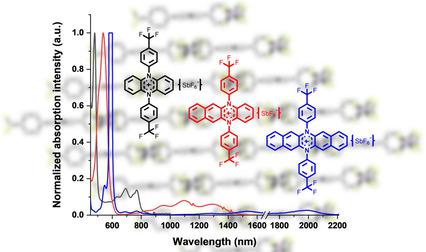
Three stable N,N’‐diarylated dihydroazaacene radical cations were prepared by oxidation of neutral N,N’‐diarylated dihydroazaacenes synthesized via palladium‐catalyzed Buchwald‐Hartwig aminations of aryl iodides with N,N’‐dihydroazaacenes. Both neutral as well as oxidized species were investigated via UV‐vis spectroscopy, single crystal analysis, and DFT calculations. All the radical cations are surprisingly stable—their absorption spectra in dichloromethane remain unchanged in ambient conditions for at least 24 hours.
中文翻译:

稳定的N,N'-二芳基二氢二氮杂双自由基自由基
通过中性的N,N'-二芳基二氢氮杂蒽酮的氧化反应制备了三个稳定的N,N'-二芳基二氢氮杂蒽自由基阳离子,该中性N,N'-二芳基化二氢氮杂蒽是通过钯催化的芳基碘化物的Buchwald-Hartwig胺与N,N'-二氢氮杂氮杂苯胺合成的。通过紫外可见光谱,单晶分析和DFT计算研究了中性和氧化性物质。所有自由基阳离子都出奇地稳定-它们在二氯甲烷中的吸收光谱在环境条件下至少24小时保持不变。
更新日期:2021-01-27
中文翻译:

稳定的N,N'-二芳基二氢二氮杂双自由基自由基
通过中性的N,N'-二芳基二氢氮杂蒽酮的氧化反应制备了三个稳定的N,N'-二芳基二氢氮杂蒽自由基阳离子,该中性N,N'-二芳基化二氢氮杂蒽是通过钯催化的芳基碘化物的Buchwald-Hartwig胺与N,N'-二氢氮杂氮杂苯胺合成的。通过紫外可见光谱,单晶分析和DFT计算研究了中性和氧化性物质。所有自由基阳离子都出奇地稳定-它们在二氯甲烷中的吸收光谱在环境条件下至少24小时保持不变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号