Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Association of Fluorescent Protein Pairs and Its Significant Impact on Fluorescence and Energy Transfer
Advanced Science ( IF 14.3 ) Pub Date : 2020-11-23 , DOI: 10.1002/advs.202003167 Jacob R Pope 1 , Rachel L Johnson 1 , W David Jamieson 2 , Harley L Worthy 1, 3 , Senthilkumar Kailasam 4, 5 , Rochelle D Ahmed 1 , Ismail Taban 1 , Husam Sabah Auhim 1, 6 , Daniel W Watkins 1, 7 , Pierre J Rizkallah 8 , Oliver K Castell 2 , D Dafydd Jones 1
Advanced Science ( IF 14.3 ) Pub Date : 2020-11-23 , DOI: 10.1002/advs.202003167 Jacob R Pope 1 , Rachel L Johnson 1 , W David Jamieson 2 , Harley L Worthy 1, 3 , Senthilkumar Kailasam 4, 5 , Rochelle D Ahmed 1 , Ismail Taban 1 , Husam Sabah Auhim 1, 6 , Daniel W Watkins 1, 7 , Pierre J Rizkallah 8 , Oliver K Castell 2 , D Dafydd Jones 1
Affiliation
|
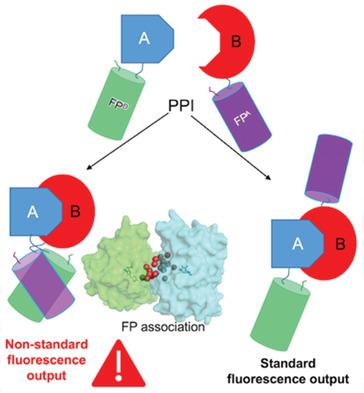
Fluorescent proteins (FPs) are commonly used in pairs to monitor dynamic biomolecular events through changes in proximity via distance dependent processes such as Förster resonance energy transfer (FRET). The impact of FP association is assessed by predicting dimerization sites in silico and stabilizing the dimers by bio‐orthogonal covalent linkages. In each tested case dimerization changes inherent fluorescence, including FRET. GFP homodimers demonstrate synergistic behavior with the dimer being brighter than the sum of the monomers. The homodimer structure reveals the chromophores are close with favorable transition dipole alignments and a highly solvated interface. Heterodimerization (GFP with Venus) results in a complex with ≈87% FRET efficiency, significantly below the 99.7% efficiency predicted. A similar efficiency is observed when the wild‐type FPs are fused to a naturally occurring protein–protein interface system. GFP complexation with mCherry results in loss of mCherry fluorescence. Thus, simple assumptions used when monitoring interactions between proteins via FP FRET may not always hold true, especially under conditions whereby the protein–protein interactions promote FP interaction.
中文翻译:

荧光蛋白对的关联及其对荧光和能量转移的显着影响
荧光蛋白 (FP) 通常成对使用,通过福斯特共振能量转移 (FRET) 等距离相关过程的接近度变化来监测动态生物分子事件。通过在计算机中预测二聚化位点并通过生物正交共价连接稳定二聚体来评估 FP 关联的影响。在每个测试案例中,二聚化都会改变固有荧光,包括 FRET。GFP 同二聚体表现出协同行为,二聚体比单体的总和更亮。同二聚体结构揭示了发色团靠近,具有有利的过渡偶极排列和高度溶剂化的界面。异二聚化(GFP 与 Venus)产生的复合物的 FRET 效率约为 87%,显着低于预测的 99.7% 效率。当野生型 FP 与天然存在的蛋白质-蛋白质界面系统融合时,观察到类似的效率。GFP 与 mCherry 络合会导致 mCherry 荧光丧失。因此,通过 FP FRET 监测蛋白质之间的相互作用时使用的简单假设可能并不总是成立,特别是在蛋白质-蛋白质相互作用促进 FP 相互作用的情况下。
更新日期:2021-01-07
中文翻译:

荧光蛋白对的关联及其对荧光和能量转移的显着影响
荧光蛋白 (FP) 通常成对使用,通过福斯特共振能量转移 (FRET) 等距离相关过程的接近度变化来监测动态生物分子事件。通过在计算机中预测二聚化位点并通过生物正交共价连接稳定二聚体来评估 FP 关联的影响。在每个测试案例中,二聚化都会改变固有荧光,包括 FRET。GFP 同二聚体表现出协同行为,二聚体比单体的总和更亮。同二聚体结构揭示了发色团靠近,具有有利的过渡偶极排列和高度溶剂化的界面。异二聚化(GFP 与 Venus)产生的复合物的 FRET 效率约为 87%,显着低于预测的 99.7% 效率。当野生型 FP 与天然存在的蛋白质-蛋白质界面系统融合时,观察到类似的效率。GFP 与 mCherry 络合会导致 mCherry 荧光丧失。因此,通过 FP FRET 监测蛋白质之间的相互作用时使用的简单假设可能并不总是成立,特别是在蛋白质-蛋白质相互作用促进 FP 相互作用的情况下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号