Industrial Crops and Products ( IF 5.6 ) Pub Date : 2020-11-20 , DOI: 10.1016/j.indcrop.2020.113095 Kwang Ho Kim , Keunhong Jeong , Jingshun Zhuang , Hye Jin Jeong , Chang Soo Kim , Bonwook Koo , Chang Geun Yoo
|
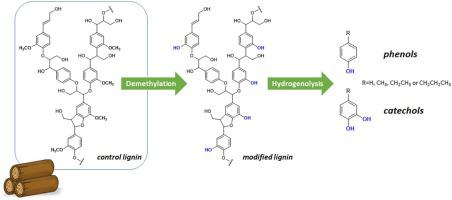
Lignin is regarded as a potential source of various aromatic compounds and chemicals, replacing petrochemicals. Catechol and its derivatives are important platform chemicals in many industrial sectors, including agrochemicals and pharmaceuticals. The global market of catechols is expected to increase with emerging demand in end-use industries. Considering that the current production of catechols relies on fossil fuels, it is critical to seek alternative renewable sources to respond to sustainability challenges. In this respect, lignin is viewed as a promising material due to its abundance and renewable character. Also, the structural similarity to catechols has made lignin a potential feedstock for the production of bio-catechols. However, the lignin-to-catechols conversion approach is still in an early stage because the intractable nature of lignin and the complex mixture of the depolymerized products make its valorization less feasible. Herein, we report a selective production of catechols via tandem conversion of lignin, namely, demethylation followed by catalytic hydrogenolysis (DFCH). The DFCH of lignin resulted in catechol- and catechol derivatives-rich liquid, accounting for 80 % in the identified products. The reaction mechanisms were also studied by quantum calculations to provide a fundamental understanding of the conversion. The result proves that selective modification of lignin structure is a promising approach to produce specific platform chemicals, providing insights toward an efficient strategy for lignin valorization.
中文翻译:

通过脱甲基和催化氢解作用将木质素串联转化为邻苯二酚
木质素被认为是各种芳族化合物和化学品的潜在来源,可代替石油化工产品。邻苯二酚及其衍生物是许多工业部门(包括农用化学品和制药业)中重要的平台化学品。预计随着最终用途行业的新兴需求,儿茶酚的全球市场将增长。考虑到目前的邻苯二酚生产依赖化石燃料,因此寻找替代性可再生资源以应对可持续发展的挑战至关重要。在这方面,木质素由于其丰富和可再生的特性而被认为是有前途的材料。而且,与邻苯二酚的结构相似性使得木质素成为生产生物邻苯二酚的潜在原料。然而,木质素向邻苯二酚的转化方法仍处于早期阶段,因为木质素的顽固性质和解聚产物的复杂混合物使得其增值作用不可行。在本文中,我们报道了通过木质素的串联转化,即脱甲基,然后催化氢解(DFCH),选择性生产邻苯二酚。木质素的DFCH生成富含儿茶酚和邻苯二酚衍生物的液体,占所鉴定产品的80%。还通过量子计算研究了反应机理,以提供对转化的基本了解。结果证明,木质素结构的选择性修饰是生产特定平台化学品的一种有前途的方法,为木质素增值的有效策略提供了见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号