Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of the A-site cation over spinel AMn2O4 (A = Cu2+, Ni2+, Zn2+) for toluene combustion: Enhancement of the synergy and the oxygen activation ability
Fuel ( IF 6.7 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.fuel.2020.119700 Yongzhao Zhang , Zequan Zeng , Yifan Li , Yaqin Hou , Jiangliang Hu , Zhanggen Huang
Fuel ( IF 6.7 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.fuel.2020.119700 Yongzhao Zhang , Zequan Zeng , Yifan Li , Yaqin Hou , Jiangliang Hu , Zhanggen Huang
|
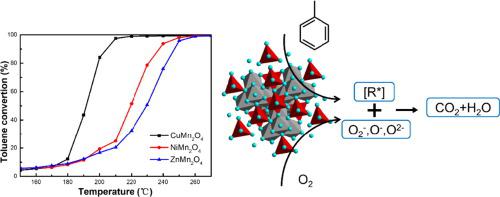
Abstract To investigate the underlying mechanism of the tetrahedral A2+ cations and the octahedral Mn3+ cations in AMn2O4 spinel catalysts for toluene oxidation, a series of structure-controlled AMn2O4 spinel catalysts were prepared through substituting A2+ and Mn3+ with Cu2+, Ni2+, Zn2+, and Fe3+, respectively. The evaluation results demonstrated that the catalytic activities arrange in the sequence of CuMn2O4 > NiMn2O4 > ZnMn2O4 > CuFe2O4, and the temperature required for 90% conversion of toluene is 205 °C for the CuMn2O4 catalyst. The physical properties of these samples were well characterized by XRD, TEM, N2 adsorption–desorption isotherms. The influences of crystal type, micro structure and specific surface area were successfully eliminated by using KIT-6 as the hard template method. The influence mechanism of A-site metal was further explored by analyzing the results of XPS, H2-TPR and O2-TPD. Results showed that the catalytic activity of the samples was highly affected by the substitution of highly electronegative A-site metal due to the enhancement of low-temperature reducibility and chemisorption oxygen activity. In particular, it was found that the incorporation of A-site metal can improve the ability of the catalyst to activate molecular state O2– to O-, which was highly related to the catalytic activity of the catalyst. The results hold guidance for the design of manganese spinel catalysts on the removal of VOCs.
中文翻译:

A位阳离子对尖晶石AMn2O4 (A = Cu2+, Ni2+, Zn2+) 甲苯燃烧的影响:增强协同作用和氧活化能力
摘要 为了研究四面体 A2+ 阳离子和八面体 Mn3+ 阳离子在 AMn2O4 尖晶石催化剂氧化甲苯中的潜在机理,通过用 Cu2+、Ni2+、Zn2+ 和 Fe3+ 取代 A2+ 和 Mn3+ 制备了一系列结构可控的 AMn2O4 尖晶石催化剂,分别。评价结果表明,催化活性按CuMn2O4>NiMn2O4>ZnMn2O4>CuFe2O4的顺序排列,CuMn2O4催化剂转化90%甲苯所需温度为205℃。这些样品的物理性质通过 XRD、TEM、N2 吸附-解吸等温线进行了很好的表征。使用KIT-6作为硬模板方法,成功消除了晶体类型、微观结构和比表面积的影响。通过对XPS、H2-TPR和O2-TPD结果的分析,进一步探讨了A位金属的影响机制。结果表明,由于低温还原性和化学吸附氧活性的增强,高负电性A位金属的取代对样品的催化活性有很大影响。特别是发现A位金属的掺入可以提高催化剂将分子态O2-活化为O-的能力,这与催化剂的催化活性高度相关。研究结果为锰尖晶石催化剂的去除VOCs的设计提供指导。结果表明,由于低温还原性和化学吸附氧活性的增强,高负电性A位金属的取代对样品的催化活性有很大影响。特别是发现A位金属的掺入可以提高催化剂将分子态O2-活化为O-的能力,这与催化剂的催化活性高度相关。研究结果为锰尖晶石催化剂的去除VOCs的设计提供指导。结果表明,由于低温还原性和化学吸附氧活性的增强,高负电性A位金属的取代对样品的催化活性有很大影响。特别是发现A位金属的掺入可以提高催化剂将分子态O2-活化为O-的能力,这与催化剂的催化活性高度相关。研究结果为锰尖晶石催化剂的去除VOCs的设计提供指导。
更新日期:2021-03-01
中文翻译:

A位阳离子对尖晶石AMn2O4 (A = Cu2+, Ni2+, Zn2+) 甲苯燃烧的影响:增强协同作用和氧活化能力
摘要 为了研究四面体 A2+ 阳离子和八面体 Mn3+ 阳离子在 AMn2O4 尖晶石催化剂氧化甲苯中的潜在机理,通过用 Cu2+、Ni2+、Zn2+ 和 Fe3+ 取代 A2+ 和 Mn3+ 制备了一系列结构可控的 AMn2O4 尖晶石催化剂,分别。评价结果表明,催化活性按CuMn2O4>NiMn2O4>ZnMn2O4>CuFe2O4的顺序排列,CuMn2O4催化剂转化90%甲苯所需温度为205℃。这些样品的物理性质通过 XRD、TEM、N2 吸附-解吸等温线进行了很好的表征。使用KIT-6作为硬模板方法,成功消除了晶体类型、微观结构和比表面积的影响。通过对XPS、H2-TPR和O2-TPD结果的分析,进一步探讨了A位金属的影响机制。结果表明,由于低温还原性和化学吸附氧活性的增强,高负电性A位金属的取代对样品的催化活性有很大影响。特别是发现A位金属的掺入可以提高催化剂将分子态O2-活化为O-的能力,这与催化剂的催化活性高度相关。研究结果为锰尖晶石催化剂的去除VOCs的设计提供指导。结果表明,由于低温还原性和化学吸附氧活性的增强,高负电性A位金属的取代对样品的催化活性有很大影响。特别是发现A位金属的掺入可以提高催化剂将分子态O2-活化为O-的能力,这与催化剂的催化活性高度相关。研究结果为锰尖晶石催化剂的去除VOCs的设计提供指导。结果表明,由于低温还原性和化学吸附氧活性的增强,高负电性A位金属的取代对样品的催化活性有很大影响。特别是发现A位金属的掺入可以提高催化剂将分子态O2-活化为O-的能力,这与催化剂的催化活性高度相关。研究结果为锰尖晶石催化剂的去除VOCs的设计提供指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号