当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Adsorption Mechanism of Resin-Activated Carbon Composite Electrode for Capacitive Deionization
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.colsurfa.2020.125935 Xiaoman Tian , Shenxu Bao , Yimin Zhang
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.colsurfa.2020.125935 Xiaoman Tian , Shenxu Bao , Yimin Zhang
|
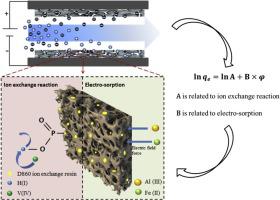
Abstract The Capacitive deionization (CDI) using D860/AC composite electrode can be used to selectively separate VO2+ from aqueous solution. An empirical equation was established to successfully describe the adsorption mechanism and selectivity of the composite electrode in CDI. By virtue of the equation, it can be speculated that the principal mechanism of Fe2+ and Al3+ adsorption on the composite electrode is the formation of electric double layer. However, the dominant adsorption mechanism for VO2+ is the formation of chemical bonds. In addition, the adsorption selectivity order of the composite electrode for different ions can be speculated based on the value of parameter A in the empirical equation: VO2+ >Fe2+ >Al3+. The effect of ions’ interaction on the adsorption characteristics was also discussed. The results showed that there was competitive adsorption between ions, and the ion hydration radius has a significant effect on the adsorption selectivity for the composite electrode in CDI.
中文翻译:

电容去离子用树脂-活性炭复合电极的选择性吸附机理
摘要 使用D860/AC复合电极的电容去离子(CDI)可用于选择性地从水溶液中分离VO2+。建立了一个经验方程,成功地描述了复合电极在 CDI 中的吸附机理和选择性。由方程可以推测,Fe2+和Al3+在复合电极上吸附的主要机理是形成双电层。然而,VO2+ 的主要吸附机制是化学键的形成。此外,根据经验方程中参数A的值,可以推测复合电极对不同离子的吸附选择性顺序:VO2+>Fe2+>Al3+。还讨论了离子相互作用对吸附特性的影响。
更新日期:2021-02-01
中文翻译:

电容去离子用树脂-活性炭复合电极的选择性吸附机理
摘要 使用D860/AC复合电极的电容去离子(CDI)可用于选择性地从水溶液中分离VO2+。建立了一个经验方程,成功地描述了复合电极在 CDI 中的吸附机理和选择性。由方程可以推测,Fe2+和Al3+在复合电极上吸附的主要机理是形成双电层。然而,VO2+ 的主要吸附机制是化学键的形成。此外,根据经验方程中参数A的值,可以推测复合电极对不同离子的吸附选择性顺序:VO2+>Fe2+>Al3+。还讨论了离子相互作用对吸附特性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号