Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-11-23 , DOI: 10.1016/j.bmcl.2020.127709 Ilya V Ozhogin 1 , Peter V Zolotukhin 2 , Eugene L Mukhanov 1 , Irina A Rostovtseva 1 , Nadezhda I Makarova 1 , Valery V Tkachev 3 , Darya K Beseda 2 , Anatoly V Metelitsa 1 , Boris S Lukyanov 1
|
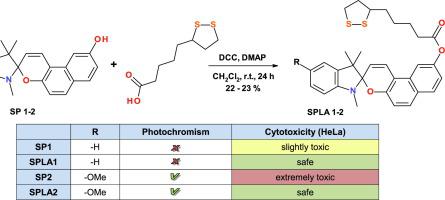
Organic photochromic compounds are attracting great interest as photoswitchable components of various bioconjugates for using in photopharmacology, targeted drug delivery and bio-imaging. Here we report on the synthesis of two novel molecular hybrids of indoline spiropyrans and alpha-lipoic acid via an esterification reaction. Preliminary photochemical studies revealed photochromic activity of 5-methoxy-substituted spirocompounds in their acetonitrile solutions. Both hybrid spiropyrans along with their parent substances in the hybrids were tested for the short-term cytotoxicity on HeLa cell cultures. The results of cytotoxicity studies showed unpredictable biocompatibility of the hybrids in comparison with the parent hydroxyl-substituted spiropyrans and α-lipoic acid, especially at the relatively high concentration of 2 mM. Using flow cytometry, we demonstrated that the both hybrids induced antioxidant response in the model cells. After the 24 h treatment, the hybrids administered at lower (500 µM) concentration caused suppressed cytosolic ROS and/or induced cellular thiols. At higher concentration, one of the hybrids demonstrated properties qualitatively similar to alpha-lipoic acid, yet far more strong. Together, flow cytometry results suggested that both hybrids of spiropyrans possess emergent biochemical and signaling antioxidant properties, exceeding those of alpha-lipoic acid.
中文翻译:

吲哚啉螺吡喃和α-硫辛酸作为潜在光药物的新型分子杂合体:合成,结构,光致变色和生物学特性
作为用于光药理学,靶向药物递送和生物成像的各种生物共轭物的可光转换组分,有机光致变色化合物引起了极大的兴趣。在这里,我们二氢吲哚螺吡喃和α-硫辛酸的两种新型分子杂化物的合成报告经由酯化反应。初步的光化学研究表明,在其乙腈溶液中,5-甲氧基取代的螺化合物的光致变色活性。测试了两种杂螺旋体及其杂种中的母体对HeLa细胞培养物的短期细胞毒性。细胞毒性研究的结果表明,与亲代羟基取代的螺吡喃和α-硫辛酸相比,杂种具有不可预测的生物相容性,尤其是在2 mM的较高浓度下。使用流式细胞仪,我们证明了这两个杂种诱导模型细胞中的抗氧化反应。处理24小时后,以较低浓度(500 µM)施用的杂种引起抑制的胞质ROS和/或诱导的细胞硫醇。在更高的浓度下 其中一种杂种在质量上表现出与α-硫辛酸相似的特性,但强度更高。一起,流式细胞仪结果表明,螺吡喃的两个杂种具有新兴的生化和信号抗氧化特性,超过了α-硫辛酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号