当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural isomers of saligenin-based β2-agonists: synthesis and insight into the reaction mechanism
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-11-17 , DOI: 10.1039/d0ob02095h Anamarija KneŽević 1 , Jurica Novak , Anita Bosak , Marijana Vinković
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-11-17 , DOI: 10.1039/d0ob02095h Anamarija KneŽević 1 , Jurica Novak , Anita Bosak , Marijana Vinković
Affiliation
|
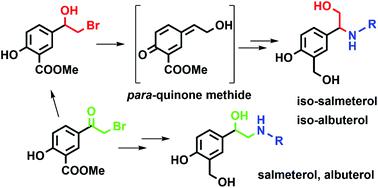
Salmeterol and albuterol are well-known β2-adenoreceptor agonists widely used in the treatment of inflammatory respiratory diseases, such as bronchial asthma and chronic obstructive pulmonary disease. Here we report the preparation of structural isomers of salmeterol and albuterol, which can be obtained from the same starting material as the corresponding β2-agonists, depending on the synthetic approach employed. Using 1D and various 2D NMR measurements, we determined that the structure of prepared isomers holds the β-aryl-β-aminoethanol moiety, in contrast to the α-aryl-β-aminoethanol moiety found in salmeterol and albuterol. We investigated the reaction of β-halohydrin and amines responsible for the formation of β-aryl-β-amino alcohol – both experimentally and using computational methods. The structure of β-halohydrin with the methyl salicylate moiety imposes the course of the reaction. The solvent plays a relevant, yet ambiguous role in the direction of the reaction, while the strength of the base influences the reaction yield and isomer ratio in a more evident way. Using computational methods, we have shown that the most probable reaction intermediate responsible for the formation of the unexpected isomer is the corresponding para-quinone methide, which can be formed due to phenol present in the methyl salicylate moiety. After successful preparation of albuterol and salmeterol isomers, we tested their inhibition potency to human acetylcholinesterase (AChE) and usual and atypical butyrylcholinesterase (BChE). Kinetic studies revealed that both isomers are low-potency reversible inhibitors of human cholinesterases.
中文翻译:

基于水杨苷的β2-激动剂的结构异构体:合成和反应机制的洞察
沙美特罗和沙丁胺醇是众所周知的β2-腺受体激动剂,广泛用于治疗炎症性呼吸道疾病,例如支气管哮喘和慢性阻塞性肺病。在这里,我们报道了沙美特罗和沙丁胺醇结构异构体的制备,它们可以从与相应的β2-激动剂相同的起始材料中获得,具体取决于所采用的合成方法。使用一维和各种二维核磁共振测量,我们确定所制备的异构体的结构含有β-芳基-β-氨基乙醇部分,与沙美特罗和沙丁胺醇中发现的α-芳基-β-氨基乙醇部分相反。我们通过实验和计算方法研究了 β-卤代醇和胺的反应,该反应负责形成 β-芳基-β-氨基醇。具有水杨酸甲酯部分的β-卤代醇的结构决定了反应的进程。溶剂在反应方向上起着相关但模糊的作用,而碱的强度以更明显的方式影响反应产率和异构体比例。使用计算方法,我们已经表明,最有可能导致意外异构体形成的反应中间体是相应的对醌甲基化物,其可以由于水杨酸甲酯部分中存在的苯酚而形成。成功制备沙丁胺醇和沙美特罗异构体后,我们测试了它们对人乙酰胆碱酯酶(AChE)以及普通和非典型丁酰胆碱酯酶(BChE)的抑制效力。动力学研究表明,两种异构体都是人类胆碱酯酶的低效可逆抑制剂。
更新日期:2020-11-22
中文翻译:

基于水杨苷的β2-激动剂的结构异构体:合成和反应机制的洞察
沙美特罗和沙丁胺醇是众所周知的β2-腺受体激动剂,广泛用于治疗炎症性呼吸道疾病,例如支气管哮喘和慢性阻塞性肺病。在这里,我们报道了沙美特罗和沙丁胺醇结构异构体的制备,它们可以从与相应的β2-激动剂相同的起始材料中获得,具体取决于所采用的合成方法。使用一维和各种二维核磁共振测量,我们确定所制备的异构体的结构含有β-芳基-β-氨基乙醇部分,与沙美特罗和沙丁胺醇中发现的α-芳基-β-氨基乙醇部分相反。我们通过实验和计算方法研究了 β-卤代醇和胺的反应,该反应负责形成 β-芳基-β-氨基醇。具有水杨酸甲酯部分的β-卤代醇的结构决定了反应的进程。溶剂在反应方向上起着相关但模糊的作用,而碱的强度以更明显的方式影响反应产率和异构体比例。使用计算方法,我们已经表明,最有可能导致意外异构体形成的反应中间体是相应的对醌甲基化物,其可以由于水杨酸甲酯部分中存在的苯酚而形成。成功制备沙丁胺醇和沙美特罗异构体后,我们测试了它们对人乙酰胆碱酯酶(AChE)以及普通和非典型丁酰胆碱酯酶(BChE)的抑制效力。动力学研究表明,两种异构体都是人类胆碱酯酶的低效可逆抑制剂。









































 京公网安备 11010802027423号
京公网安备 11010802027423号