当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distribution of alkali cations near the Cu (111) surface in aqueous solution
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-11-12 , DOI: 10.1039/d0ta08965f Cong Xi 1, 2, 3, 4, 5 , Fan Zheng 6, 7, 8, 9 , Guoping Gao 6, 7, 8, 9 , Meng Ye 6, 7, 8, 9 , Cunku Dong 1, 2, 3, 4, 5 , Xi-Wen Du 1, 2, 3, 4, 5 , Lin-Wang Wang 6, 7, 8, 9, 10
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-11-12 , DOI: 10.1039/d0ta08965f Cong Xi 1, 2, 3, 4, 5 , Fan Zheng 6, 7, 8, 9 , Guoping Gao 6, 7, 8, 9 , Meng Ye 6, 7, 8, 9 , Cunku Dong 1, 2, 3, 4, 5 , Xi-Wen Du 1, 2, 3, 4, 5 , Lin-Wang Wang 6, 7, 8, 9, 10
Affiliation
|
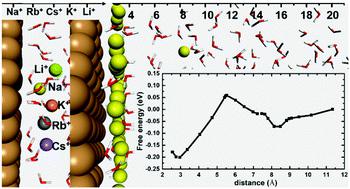
It has been widely realized, in aqueous solution, that metal cations near a catalytic surface in the Helmholtz layer can have a significant influence on catalytic reduction processes, from CO2 reduction to H2 generation. However, the exact nature of the cation distribution, the free energy profile, and local coordination with water molecules, as well as the electrode potential dependence are still not known. For example, it is known that water molecules can form some layer structures above the surface. Are the cations in some particular positions in the water layer? What is the free energy profile of a cation as a function of its position? What is the cation density at the surface for a given bulk concentration? How do their energy profiles depend on the electrode potential? What is the trend for different cations due to their size and chemistry difference? Answering these questions is essential to understand the role of cations in aqueous based catalytic processes. In this work, using ab initio molecular dynamics (AIMD) simulations with explicit water, we provide a systematic study of the above questions for the series of alkali cations (Li+, Na+, K+, Rb+ and Cs+) on both neutral and charged Cu (111) electrodes (corresponding to electrode potentials from −2.16 V to 1.56 V vs. SHE). The results indicate that the alkali cations will remain near the electrode–electrolyte interface over a wide potential range (from −2.16 V to 1.37 V). The free energy profile obtained from thermodynamics integration shows that Na+ likes to remain near the interface, and it prefers the odd layer of the water structure. A simple model is provided to explain the underlying reason for such behavior. We also find that the surface concentration can be very high for a moderate bulk cation concentration, indicating a possible strong cation role in the catalytic process.
中文翻译:

水溶液中Cu(111)表面附近碱金属阳离子的分布
在水溶液中,人们已经广泛认识到,亥姆霍兹层中催化表面附近的金属阳离子会对催化还原过程(从CO 2还原为H 2)产生重大影响。代。然而,阳离子分布的确切性质,自由能分布,与水分子的局部配位以及电极电势依赖性仍然未知。例如,已知水分子可以在表面上方形成一些层结构。阳离子是否在水层的某些特定位置?阳离子的自由能曲线是其位置的函数吗?对于给定的体积浓度,表面的阳离子密度是多少?它们的能量分布如何取决于电极电势?由于阳离子的大小和化学差异,不同阳离子的趋势如何?回答这些问题对于理解阳离子在水基催化过程中的作用至关重要。在这项工作中,使用从头开始用显性水进行分子动力学(AIMD)模拟,我们对中性和带电Cu(111)上的一系列碱性阳离子(Li +,Na +,K +,Rb +和Cs +)的上述问题提供了系统的研究。电极(对应于-2.16 V至1.56 V vs. SHE的电极电势)。结果表明,碱阳离子将在很宽的电位范围(-2.16 V至1.37 V)内保留在电极-电解质界面附近。从热力学积分获得的自由能曲线表明,Na +喜欢保留在界面附近,它更喜欢水结构的奇数层。提供了一个简单的模型来解释这种行为的根本原因。我们还发现,对于中等体积的阳离子浓度,表面浓度可能很高,这表明在催化过程中可能存在强阳离子作用。
更新日期:2020-11-22
中文翻译:

水溶液中Cu(111)表面附近碱金属阳离子的分布
在水溶液中,人们已经广泛认识到,亥姆霍兹层中催化表面附近的金属阳离子会对催化还原过程(从CO 2还原为H 2)产生重大影响。代。然而,阳离子分布的确切性质,自由能分布,与水分子的局部配位以及电极电势依赖性仍然未知。例如,已知水分子可以在表面上方形成一些层结构。阳离子是否在水层的某些特定位置?阳离子的自由能曲线是其位置的函数吗?对于给定的体积浓度,表面的阳离子密度是多少?它们的能量分布如何取决于电极电势?由于阳离子的大小和化学差异,不同阳离子的趋势如何?回答这些问题对于理解阳离子在水基催化过程中的作用至关重要。在这项工作中,使用从头开始用显性水进行分子动力学(AIMD)模拟,我们对中性和带电Cu(111)上的一系列碱性阳离子(Li +,Na +,K +,Rb +和Cs +)的上述问题提供了系统的研究。电极(对应于-2.16 V至1.56 V vs. SHE的电极电势)。结果表明,碱阳离子将在很宽的电位范围(-2.16 V至1.37 V)内保留在电极-电解质界面附近。从热力学积分获得的自由能曲线表明,Na +喜欢保留在界面附近,它更喜欢水结构的奇数层。提供了一个简单的模型来解释这种行为的根本原因。我们还发现,对于中等体积的阳离子浓度,表面浓度可能很高,这表明在催化过程中可能存在强阳离子作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号