当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent functionalization of terminal alkynes enabled alkynylative [5+1] benzannulation of 3-acetoxy-1,4-enynes
Chemical Communications ( IF 4.3 ) Pub Date : 2020-11-14 , DOI: 10.1039/d0cc06793h Li-Jun Wu 1, 2, 3 , Liang-Feng Yang 1, 2, 3, 4, 5 , Gui-Fen Lv 1, 2, 3 , Jin-Heng Li 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2020-11-14 , DOI: 10.1039/d0cc06793h Li-Jun Wu 1, 2, 3 , Liang-Feng Yang 1, 2, 3, 4, 5 , Gui-Fen Lv 1, 2, 3 , Jin-Heng Li 1, 2, 3, 4, 5
Affiliation
|
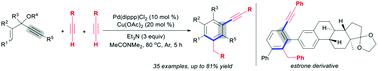
We here describe an alkynylative [5+1] benzannulation of 3-acetoxy-1,4-enynes with terminal alkynes, which enables both the construction of a benzene ring skeleton and intermolecular incorporation of an alkynyl group in a single reaction using Pd and Cu cooperative catalysts. The method represents efficient access to internal aryl alkynes through divergent functionalization of two terminal alkyne components: one alkyne serves as the one-carbon unit to realize the [5+1] benzannulation and the other alkyne as a nucleophile terminates the reaction.
中文翻译:

末端炔烃的不同官能化可实现3-乙酰氧基-1,4-烯炔的炔基化[5 + 1]苯环
我们在这里描述了3-乙酰氧基-1,4-烯炔与末端炔烃的炔基化[5 + 1]苯环,这使得苯环骨架的构建和炔基在单个反应中使用Pd和Cu的分子间结合成为可能合作催化剂。该方法表示通过两个末端炔烃组分的不同官能化来有效地获得内部芳基炔烃:一个炔烃用作实现[5 + 1]苯并环化反应的一个碳单元,另一个炔烃作为亲核试剂终止反应。
更新日期:2020-11-22
中文翻译:

末端炔烃的不同官能化可实现3-乙酰氧基-1,4-烯炔的炔基化[5 + 1]苯环
我们在这里描述了3-乙酰氧基-1,4-烯炔与末端炔烃的炔基化[5 + 1]苯环,这使得苯环骨架的构建和炔基在单个反应中使用Pd和Cu的分子间结合成为可能合作催化剂。该方法表示通过两个末端炔烃组分的不同官能化来有效地获得内部芳基炔烃:一个炔烃用作实现[5 + 1]苯并环化反应的一个碳单元,另一个炔烃作为亲核试剂终止反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号