当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improving the Stability of EGFR Inhibitor Cobalt(III) Prodrugs
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-21 , DOI: 10.1021/acs.inorgchem.0c03083 Marlene Mathuber 1 , Hemma Schueffl 2 , Orsolya Dömötör 3, 4 , Claudia Karnthaler 1 , Éva A Enyedy 3, 4 , Petra Heffeter 2, 5 , Bernhard K Keppler 1, 5 , Christian R Kowol 1, 5
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-21 , DOI: 10.1021/acs.inorgchem.0c03083 Marlene Mathuber 1 , Hemma Schueffl 2 , Orsolya Dömötör 3, 4 , Claudia Karnthaler 1 , Éva A Enyedy 3, 4 , Petra Heffeter 2, 5 , Bernhard K Keppler 1, 5 , Christian R Kowol 1, 5
Affiliation
|
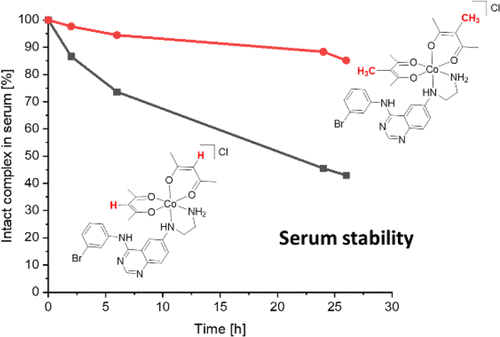
Although tyrosine kinase inhibitors (TKIs) have revolutionized cancer therapy in the past two decades, severe drawbacks such as strong adverse effects and drug resistance limit their clinical application. Prodrugs represent a valuable approach to overcoming these disadvantages by administration of an inactive drug with tumor-specific activation. We have recently shown that hypoxic prodrug activation is a promising strategy for a cobalt(III) complex bearing a TKI of the epidermal growth factor receptor (EGFR). The aim of this study was the optimization of the physicochemical properties and enhancement of the stability of this compound class. Therefore, we synthesized a series of novel derivatives to investigate the influence of the electron-donating properties of methyl substituents at the metal-chelating moiety of the EGFR inhibitor and/or the ancillary acetylacetonate (acac) ligand. To understand the effect of the different methylations on the redox properties, the newly synthesized complexes were analyzed by cyclic voltammetry and their behavior was studied in the presence of natural low-molecular weight reducing agents. Furthermore, it was proven that reduction to cobalt(II) resulted in a lower stability of the complexes and subsequent release of the coordinated TKI ligand. Moreover, the stability of the cobalt(III) prodrugs was investigated in blood serum as well as in cell culture by diverse cell and molecular biological methods. These analyses revealed that the complexes bearing the methylated acac ligand are characterized by distinctly enhanced stability. Finally, the cytotoxic activity of all new compounds was tested in cell culture under normoxic and various hypoxic conditions, and their prodrug nature could be correlated convincingly with the stability data. In summary, the performed chemical modifications resulted in new cobalt(III) prodrugs with strongly improved stabilities together with retained hypoxia-activatable properties.
中文翻译:

改善EGFR抑制剂钴(III)前药的稳定性
尽管酪氨酸激酶抑制剂(TKIs)在过去的二十年中已经彻底改变了癌症治疗方法,但严重的缺点(如强烈的副作用和耐药性)限制了它们的临床应用。前药是通过给予具有肿瘤特异性活化作用的非活性药物来克服这些缺点的有价值的方法。我们最近显示,低氧前药激活是一种带有表皮生长因子受体(EGFR)TKI的钴(III)复合物的有前途的策略。这项研究的目的是优化该化合物类别的理化性质并增强其稳定性。因此,我们合成了一系列新颖的衍生物,以研究在EGFR抑制剂和/或辅助乙酰丙酮酸(acac)配体的金属螯合部分上,甲基取代基的供电子性质的影响。为了了解不同甲基化对氧化还原特性的影响,通过循环伏安法分析了新合成的配合物,并在天然低分子量还原剂存在下研究了它们的行为。此外,已证明还原为钴(II)导致复合物的稳定性降低,并随后释放出配位的TKI配体。此外,通过多种细胞和分子生物学方法研究了钴(III)前药在血清以及细胞培养物中的稳定性。这些分析表明,带有甲基化的acac配体的复合物具有明显增强的稳定性。最后,在常氧和各种低氧条件下,在细胞培养物中测试了所有新化合物的细胞毒性活性,其前药性质可与稳定性数据令人信服地相关。总之,所进行的化学修饰产生了新的钴(III)前药,其稳定性大大增强,同时保留了可缺氧的活性。
更新日期:2020-12-07
中文翻译:

改善EGFR抑制剂钴(III)前药的稳定性
尽管酪氨酸激酶抑制剂(TKIs)在过去的二十年中已经彻底改变了癌症治疗方法,但严重的缺点(如强烈的副作用和耐药性)限制了它们的临床应用。前药是通过给予具有肿瘤特异性活化作用的非活性药物来克服这些缺点的有价值的方法。我们最近显示,低氧前药激活是一种带有表皮生长因子受体(EGFR)TKI的钴(III)复合物的有前途的策略。这项研究的目的是优化该化合物类别的理化性质并增强其稳定性。因此,我们合成了一系列新颖的衍生物,以研究在EGFR抑制剂和/或辅助乙酰丙酮酸(acac)配体的金属螯合部分上,甲基取代基的供电子性质的影响。为了了解不同甲基化对氧化还原特性的影响,通过循环伏安法分析了新合成的配合物,并在天然低分子量还原剂存在下研究了它们的行为。此外,已证明还原为钴(II)导致复合物的稳定性降低,并随后释放出配位的TKI配体。此外,通过多种细胞和分子生物学方法研究了钴(III)前药在血清以及细胞培养物中的稳定性。这些分析表明,带有甲基化的acac配体的复合物具有明显增强的稳定性。最后,在常氧和各种低氧条件下,在细胞培养物中测试了所有新化合物的细胞毒性活性,其前药性质可与稳定性数据令人信服地相关。总之,所进行的化学修饰产生了新的钴(III)前药,其稳定性大大增强,同时保留了可缺氧的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号