当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Metallocene Analogues of the Phenethylamine and Tetrahydroisoquinoline Scaffolds via Regioselective Ring Opening of 2‐Aryl‐N‐sulfonyl Aziridines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-20 , DOI: 10.1002/adsc.202001210 Silvia González‐Pelayo 1 , Olaya Bernardo 1 , Javier Borge 2 , Luis A. López 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-11-20 , DOI: 10.1002/adsc.202001210 Silvia González‐Pelayo 1 , Olaya Bernardo 1 , Javier Borge 2 , Luis A. López 1
Affiliation
|
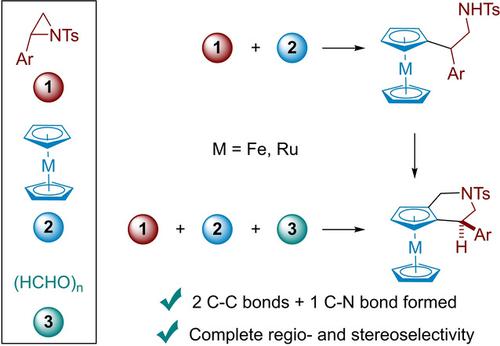
The Lewis (or Brønsted) acid‐catalyzed reaction of 2‐aryl‐N‐sulfonyl aziridines with ferrocene and ruthenocene provided new amino‐functionalized metallocene derivatives arising from a regioselective ring opening of the aziridine. The functionalized metallocene derivatives available by this methodology are suitable precursors for the stereoselective synthesis of metallocene analogues of the relevant tetrahydroisoquinoline motif by a Pictet‐Spengler type reaction. These isoquinoline analogues are also accessible by a TfOH‐catalyzed three‐component reaction of 2‐aryl‐N‐sulfonyl aziridines, ferrocene (or ruthenocene) and formaldehyde.
中文翻译:

通过2-芳基-N-磺酰基氮丙啶的区域选择性开环合成苯乙胺和四氢异喹啉骨架的茂金属类似物
2芳基N磺酰基氮丙啶与二茂铁和钌茂茂的Lewis(或Brønsted)酸催化反应提供了新的氨基官能化的茂金属衍生物,这是由于氮丙啶的区域选择性开环而产生的。通过该方法可获得的功能化茂金属衍生物是适合的前体,可通过Pictet-Spengler型反应立体合成相关四氢异喹啉基序的茂金属类似物。这些异喹啉类似物也可通过TfOH催化的2芳基N N磺酰基氮丙啶,二茂铁(或钌茂铁)和甲醛的三组分反应获得。
更新日期:2020-11-20
中文翻译:

通过2-芳基-N-磺酰基氮丙啶的区域选择性开环合成苯乙胺和四氢异喹啉骨架的茂金属类似物
2芳基N磺酰基氮丙啶与二茂铁和钌茂茂的Lewis(或Brønsted)酸催化反应提供了新的氨基官能化的茂金属衍生物,这是由于氮丙啶的区域选择性开环而产生的。通过该方法可获得的功能化茂金属衍生物是适合的前体,可通过Pictet-Spengler型反应立体合成相关四氢异喹啉基序的茂金属类似物。这些异喹啉类似物也可通过TfOH催化的2芳基N N磺酰基氮丙啶,二茂铁(或钌茂铁)和甲醛的三组分反应获得。











































 京公网安备 11010802027423号
京公网安备 11010802027423号