当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A key cytosolic iron–sulfur cluster synthesis protein localizes to the mitochondrion of Toxoplasma gondii
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-22 , DOI: 10.1111/mmi.14651 Yi Tong Vincent Aw 1 , Azadeh Seidi 1 , Jenni A Hayward 1 , Jiwon Lee 2 , F Victor Makota 1 , Melanie Rug 2 , Giel G van Dooren 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-22 , DOI: 10.1111/mmi.14651 Yi Tong Vincent Aw 1 , Azadeh Seidi 1 , Jenni A Hayward 1 , Jiwon Lee 2 , F Victor Makota 1 , Melanie Rug 2 , Giel G van Dooren 1
Affiliation
|
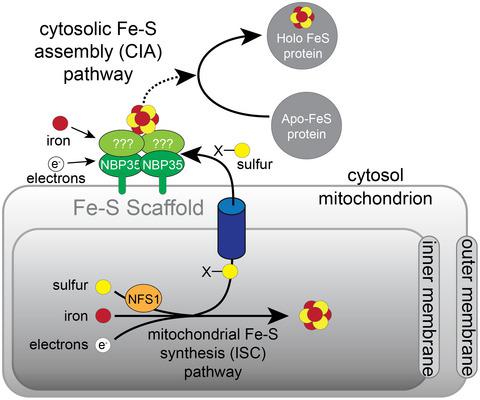
Iron–sulfur (Fe-S) clusters are prosthetic groups on proteins that function in a range of enzymatic and electron transfer reactions. Fe-S cluster synthesis is essential for the survival of all eukaryotes. Independent Fe-S cluster biosynthesis pathways occur in the mitochondrion, plastid, and cytosolic compartments of eukaryotic cells. Little is known about the cytosolic Fe-S cluster biosynthesis in apicomplexan parasites, the causative agents of diseases such as malaria and toxoplasmosis. NBP35 serves as a key scaffold protein on which cytosolic Fe-S clusters assemble, and has a cytosolic localization in most eukaryotes studied thus far. Unexpectedly, we found that the NBP35 homolog of the apicomplexan Toxoplasma gondii (TgNBP35) localizes to the outer mitochondrial membrane, with mitochondrial targeting mediated by an N-terminal transmembrane domain. We demonstrate that TgNBP35 is critical for parasite proliferation, but that, despite its mitochondrial localization, it is not required for Fe-S cluster synthesis in the mitochondrion. Instead, we establish that TgNBP35 is important for the biogenesis of cytosolic Fe-S proteins. Our data are consistent with TgNBP35 playing a central and specific role in cytosolic Fe-S cluster biosynthesis, and imply that the assembly of cytosolic Fe-S clusters occurs on the cytosolic face of the outer mitochondrial membrane in these parasites.
中文翻译:

一种关键的细胞溶质铁硫簇合成蛋白定位于弓形虫线粒体
铁硫 (Fe-S) 簇是蛋白质上的辅基,在一系列酶促和电子转移反应中起作用。Fe-S簇合成对于所有真核生物的生存都是必不可少的。独立的 Fe-S 簇生物合成途径发生在真核细胞的线粒体、质体和胞质区室中。人们对顶复体寄生虫(疟疾和弓形体病等疾病的病原体)的胞质 Fe-S 簇生物合成知之甚少。NBP35 作为一种关键的支架蛋白,细胞质 Fe-S 簇在其上组装,并且在迄今为止研究的大多数真核生物中具有细胞质定位。出乎意料的是,我们发现顶复体弓形虫的 NBP35 同源物(TgNBP35) 定位于线粒体外膜,线粒体靶向由 N 端跨膜结构域介导。我们证明Tg NBP35 对寄生虫增殖至关重要,但尽管它位于线粒体,但它并不是线粒体中 Fe-S 簇合成所必需的。相反,我们确定Tg NBP35 对胞质 Fe-S 蛋白的生物发生很重要。我们的数据与Tg NBP35 在细胞质 Fe-S 簇生物合成中发挥核心和特定作用一致,并暗示细胞质 Fe-S 簇的组装发生在这些寄生虫的外线粒体膜的细胞质面上。
更新日期:2020-11-22
中文翻译:

一种关键的细胞溶质铁硫簇合成蛋白定位于弓形虫线粒体
铁硫 (Fe-S) 簇是蛋白质上的辅基,在一系列酶促和电子转移反应中起作用。Fe-S簇合成对于所有真核生物的生存都是必不可少的。独立的 Fe-S 簇生物合成途径发生在真核细胞的线粒体、质体和胞质区室中。人们对顶复体寄生虫(疟疾和弓形体病等疾病的病原体)的胞质 Fe-S 簇生物合成知之甚少。NBP35 作为一种关键的支架蛋白,细胞质 Fe-S 簇在其上组装,并且在迄今为止研究的大多数真核生物中具有细胞质定位。出乎意料的是,我们发现顶复体弓形虫的 NBP35 同源物(TgNBP35) 定位于线粒体外膜,线粒体靶向由 N 端跨膜结构域介导。我们证明Tg NBP35 对寄生虫增殖至关重要,但尽管它位于线粒体,但它并不是线粒体中 Fe-S 簇合成所必需的。相反,我们确定Tg NBP35 对胞质 Fe-S 蛋白的生物发生很重要。我们的数据与Tg NBP35 在细胞质 Fe-S 簇生物合成中发挥核心和特定作用一致,并暗示细胞质 Fe-S 簇的组装发生在这些寄生虫的外线粒体膜的细胞质面上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号