当前位置:
X-MOL 学术
›
J. Anal. Appl. Pyrol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transformation of nitrogen, sulfur and chlorine during waste tire pyrolysis
Journal of Analytical and Applied Pyrolysis ( IF 6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jaap.2020.104987 Zhanjun Cheng , Miao Li , Jiantao Li , Fawei Lin , Wenchao Ma , Beibei Yan , Guanyi Chen
Journal of Analytical and Applied Pyrolysis ( IF 6 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jaap.2020.104987 Zhanjun Cheng , Miao Li , Jiantao Li , Fawei Lin , Wenchao Ma , Beibei Yan , Guanyi Chen
|
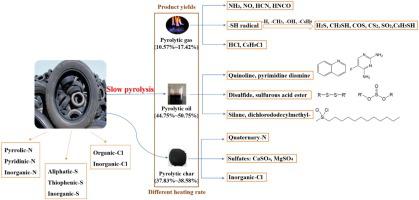
Abstract The transformation of N/S/Cl during pyrolysis of waste tire were investigated by Thermogravimetry-Mass Spectrum (TG-MS) and flow tube furnace reactor. The pyrolysis of waste tire included four stages, i.e., dehydration below 200 ℃, decomposition of tire additives at 200∼300 ℃, degradation of natural rubber at 300∼420 ℃, and cracking of synthetic rubber at 420∼500 ℃. The activation energy Eα were calculated according to the Coats-Redfern integral method, ca. 154.72∼158.23 and 200.46∼231.58 kJ/mol for degradation of natural rubber and synthetic rubber, respectively. Most of nitrogen (60.32∼67.78 wt.%), sulfur (56.73∼62.38 wt.%), and chlorine (58.60∼64.92 wt.%) were remained in the pyrolytic char. For the pyrolytic oil composition, expect for alkanes, alkenes, aromatic hydrocarbons, and oxygenates, the S-containing disulfide and sulfurous acid ester, N-containing quinoline and pyrimidine diamine, and Cl-containing silane, dichlorododecylmethyl- were detected. The nitrogen, sulfur, and chlorine in pyrolytic gas had diverse types. The N-containing pollutants mainly derived from inorganic ammonium and heterocyclic-N. NH3 had a wide releasing temperature range, while NO, HCN, and HNCO were mainly generated at 300∼600 °C. The C-S and -SH radicals mainly contributed to S-containing pollutants, i.e., H2S, COS, CS2, SO2, CH3SH, and C6H5SH. The Cl-containing pollutants exhibited dominant release within the temperature range of 300∼600 °C. Overall, higher heating rate promoted gas emissions, especially for NO, HCN, CH3SH, and HCl. This article provides basic knowledge on hazardous N/S/Cl transformation in solid char, liquid oil, and gas during pyrolysis of waste tire that will provide valuable information for future control technologies of pollutants emission.
中文翻译:

废轮胎热解过程中氮、硫、氯的转化
摘要 采用热重-质谱(TG-MS)和流管炉反应器研究了废轮胎热解过程中N/S/Cl的转化。废轮胎的热解包括200℃以下脱水、200~300℃轮胎添加剂分解、300~420℃天然橡胶降解、420~500℃合成橡胶裂解四个阶段。活化能 Eα 根据 Coats-Redfern 积分法计算,约。降解天然橡胶和合成橡胶分别为 154.72∼158.23 和 200.46∼231.58 kJ/mol。大部分氮(60.32~67.78 wt.%)、硫(56.73~62.38 wt.%)和氯(58.60~64.92 wt.%)留在热解焦炭中。对于热解油成分,除了烷烃、烯烃、芳香烃和含氧化合物,检测出含硫二硫化物和亚硫酸酯,含氮喹啉和嘧啶二胺,含氯硅烷,二氯十二烷基甲基-。热解气中的氮、硫、氯有多种类型。含氮污染物主要来源于无机铵和杂环氮。NH3 的释放温度范围较宽,而 NO、HCN 和 HNCO 主要在 300~600 ℃下生成。CS和-SH自由基主要对含S污染物产生贡献,即H2S、COS、CS2、SO2、CH3SH和C6H5SH。含Cl污染物在300~600℃的温度范围内表现出显着释放。总体而言,较高的加热速率促进了气体排放,尤其是 NO、HCN、CH3SH 和 HCl。本文提供了有关固体炭、液体油中有害 N/S/Cl 转化的基础知识
更新日期:2021-01-01
中文翻译:

废轮胎热解过程中氮、硫、氯的转化
摘要 采用热重-质谱(TG-MS)和流管炉反应器研究了废轮胎热解过程中N/S/Cl的转化。废轮胎的热解包括200℃以下脱水、200~300℃轮胎添加剂分解、300~420℃天然橡胶降解、420~500℃合成橡胶裂解四个阶段。活化能 Eα 根据 Coats-Redfern 积分法计算,约。降解天然橡胶和合成橡胶分别为 154.72∼158.23 和 200.46∼231.58 kJ/mol。大部分氮(60.32~67.78 wt.%)、硫(56.73~62.38 wt.%)和氯(58.60~64.92 wt.%)留在热解焦炭中。对于热解油成分,除了烷烃、烯烃、芳香烃和含氧化合物,检测出含硫二硫化物和亚硫酸酯,含氮喹啉和嘧啶二胺,含氯硅烷,二氯十二烷基甲基-。热解气中的氮、硫、氯有多种类型。含氮污染物主要来源于无机铵和杂环氮。NH3 的释放温度范围较宽,而 NO、HCN 和 HNCO 主要在 300~600 ℃下生成。CS和-SH自由基主要对含S污染物产生贡献,即H2S、COS、CS2、SO2、CH3SH和C6H5SH。含Cl污染物在300~600℃的温度范围内表现出显着释放。总体而言,较高的加热速率促进了气体排放,尤其是 NO、HCN、CH3SH 和 HCl。本文提供了有关固体炭、液体油中有害 N/S/Cl 转化的基础知识



























 京公网安备 11010802027423号
京公网安备 11010802027423号