当前位置:
X-MOL 学术
›
Int. J. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal decomposition mechanism of allyltrichlorosilane and allyltrimethylsilane
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.ijms.2020.116476 Kuanliang Shao , Yi Tian , Jingsong Zhang
International Journal of Mass Spectrometry ( IF 1.6 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.ijms.2020.116476 Kuanliang Shao , Yi Tian , Jingsong Zhang
|
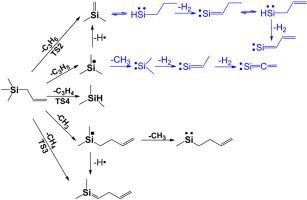
Abstract Thermal decomposition of allyltrichlorosilane and allyltrimethylsilane at temperatures up to 1340 K were studied using flash pyrolysis coupled with molecular beam sampling and vacuum ultraviolet single-photon ionization time-of-flight mass spectrometry (VUV-SPI-TOFMS). The initial reaction of allyltrichlorosilane was homolysis of the Si-C bond, producing •SiCl3 and •C3H5 radicals. The •SiCl3 radical then underwent sequential Cl losses to form:SiCl2, SiCl and Si. The C3H5 radical also underwent secondary reactions, producing a series of C3Hx species (m/z = 39-42), consistent with previous studies. In the pyrolysis of allyltrimethylsilane, molecular elimination reaction forming Me2Si=CH2 + C3H6 and Si-C bond ruptures producing Me3Si• + •C3H5 and M e 2 S i ˙ C H 2 C H = C H 2 + •CH3 were identified as the main initiation decomposition steps. HSiMe3 was also identified as one of the initiation reaction products. The observed secondary reactions of Me2Si=CH2 and Me3Si• were consistent with previous studies. Possible thermal decomposition mechanism of the M e 2 S i ˙ C H 2 C H = C H 2 product was also proposed.
中文翻译:

烯丙基三氯硅烷和烯丙基三甲基硅烷的热分解机理
摘要 使用快速热解结合分子束采样和真空紫外单光子电离飞行时间质谱 (VUV-SPI-TOFMS) 研究了烯丙基三氯硅烷和烯丙基三甲基硅烷在高达 1340 K 的热分解。烯丙基三氯硅烷的初始反应是 Si-C 键的均裂,产生 •SiCl3 和 •C3H5 自由基。•SiCl3 自由基随后经历连续的 Cl 损失以形成:SiCl2、SiCl 和 Si。C3H5 自由基也经历了二次反应,产生了一系列 C3Hx 物种(m/z = 39-42),与之前的研究一致。在烯丙基三甲基硅烷的热解中,分子消除反应形成 Me2Si=CH2 + C3H6 和 Si-C 键断裂产生 Me3Si• + •C3H5 和 Me 2 Si ˙ CH 2 CH = CH 2 + •CH3 被确定为主要的引发分解脚步。HSiMe3 也被确定为引发反应产物之一。观察到的 Me2Si=CH2 和 Me3Si• 的二次反应与之前的研究一致。还提出了 Me 2 S i ˙ CH 2 CH = CH 2 产物的可能热分解机制。
更新日期:2021-02-01
中文翻译:

烯丙基三氯硅烷和烯丙基三甲基硅烷的热分解机理
摘要 使用快速热解结合分子束采样和真空紫外单光子电离飞行时间质谱 (VUV-SPI-TOFMS) 研究了烯丙基三氯硅烷和烯丙基三甲基硅烷在高达 1340 K 的热分解。烯丙基三氯硅烷的初始反应是 Si-C 键的均裂,产生 •SiCl3 和 •C3H5 自由基。•SiCl3 自由基随后经历连续的 Cl 损失以形成:SiCl2、SiCl 和 Si。C3H5 自由基也经历了二次反应,产生了一系列 C3Hx 物种(m/z = 39-42),与之前的研究一致。在烯丙基三甲基硅烷的热解中,分子消除反应形成 Me2Si=CH2 + C3H6 和 Si-C 键断裂产生 Me3Si• + •C3H5 和 Me 2 Si ˙ CH 2 CH = CH 2 + •CH3 被确定为主要的引发分解脚步。HSiMe3 也被确定为引发反应产物之一。观察到的 Me2Si=CH2 和 Me3Si• 的二次反应与之前的研究一致。还提出了 Me 2 S i ˙ CH 2 CH = CH 2 产物的可能热分解机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号