European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.ejmech.2020.113042 Johan L Å Nilsson 1 , Christophe Mallet 2 , Kiseko Shionoya 3 , Anders Blomgren 1 , Anders P Sundin 4 , Lars Grundemar 1 , Ludivine Boudieu 2 , Anders Blomqvist 3 , Alain Eschalier 2 , Ulf J Nilsson 4 , Peter M Zygmunt 5
|
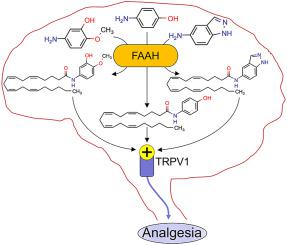
Paracetamol, one of the most widely used pain-relieving drugs, is deacetylated to 4-aminophenol (4-AP) that undergoes fatty acid amide hydrolase (FAAH)-dependent biotransformation into N-arachidonoylphenolamine (AM404), which mediates TRPV1-dependent antinociception in the brain of rodents. However, paracetamol is also converted to the liver-toxic metabolite N-acetyl-p-benzoquinone imine already at therapeutic doses, urging for safer paracetamol analogues. Primary amine analogues with chemical structures similar to paracetamol were evaluated for their propensity to undergo FAAH-dependent N-arachidonoyl conjugation into TRPV1 activators both in vitro and in vivo in rodents. The antinociceptive and antipyretic activity of paracetamol and primary amine analogues were examined with regard to FAAH and TRPV1 as well as if these analogues produced acute liver toxicity. 5-Amino-2-methoxyphenol (2) and 5-aminoindazole (3) displayed efficient target protein interactions with a dose-dependent antinociceptive effect in the mice formalin test, which in the second phase was dependent on FAAH and TRPV1. No hepatotoxicity of the FAAH substrates transformed into TRPV1 activators was observed. While paracetamol attenuates pyrexia via inhibition of brain cyclooxygenase, its antinociceptive FAAH substrate 4-AP was not antipyretic, suggesting separate mechanisms for the antipyretic and antinociceptive effect of paracetamol. Furthermore, compound 3 reduced fever without a brain cyclooxygenase inhibitory action. The data support our view that analgesics and antipyretics without liver toxicity can be derived from paracetamol. Thus, research into the molecular actions of paracetamol could pave the way for the discovery of analgesics and antipyretics with a better benefit-to-risk ratio.
中文翻译:

FAAH 缀合的扑热息痛类似物可诱导 TRPV1 介导的镇痛作用,且不会引起急性肝毒性
扑热息痛是最广泛使用的止痛药物之一,它被脱乙酰基化为 4-氨基苯酚 (4-AP),经过脂肪酸酰胺水解酶 (FAAH) 依赖性生物转化为N-花生四烯酰酚胺 (AM404),后者介导 TRPV1 依赖性镇痛作用在啮齿动物的大脑中。然而,扑热息痛在达到治疗剂量时也会转化为肝毒性代谢物N-乙酰基-对苯醌亚胺,因此需要更安全的扑热息痛类似物。化学结构与扑热息痛类似的伯胺类似物在啮齿类动物体内和体外进行FAAH依赖性N-花生四烯酰缀合成TRPV1激活剂的倾向进行了评估。检查扑热息痛和伯胺类似物对 FAAH 和 TRPV1 的镇痛和解热活性,以及这些类似物是否产生急性肝毒性。 5-氨基-2-甲氧基苯酚 ( 2 ) 和 5-氨基吲唑 ( 3 ) 在小鼠福尔马林测试中表现出有效的靶蛋白相互作用和剂量依赖性镇痛作用,该作用在第二阶段依赖于 FAAH 和 TRPV1。未观察到转化为 TRPV1 激活剂的 FAAH 底物有肝毒性。虽然扑热息痛通过抑制脑环氧合酶来减轻发热,但其镇痛 FAAH 底物 4-AP 并不具有解热作用,这表明扑热息痛的解热和镇痛作用具有不同的机制。此外,化合物3可以降低发烧,但没有脑环氧合酶抑制作用。这些数据支持我们的观点,即无肝毒性的镇痛药和退烧药可以从扑热息痛中提取。 因此,对扑热息痛分子作用的研究可以为发现具有更好的效益风险比的镇痛药和退热药铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号