Environmental Pollution ( IF 7.6 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.envpol.2020.116122 Wanfeng Wang , Panqing Yang , Yanling Guo , Haoran Ji , Fang Liang
|
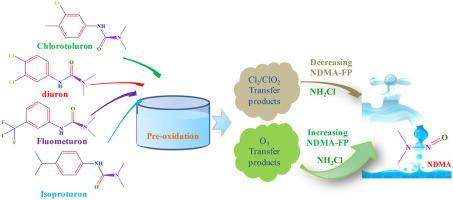
Four phenylurea herbicides (PUHs) were assessed for degradation and transformation into N-nitrosodimethylamine (NDMA) under three oxidation conditions (chlorine (Cl2), chlorine dioxide (ClO2), and ozone (O3)) from an aqueous solution. Removal ratios correlated with the numbers of halogen elements contained in PUHs (isoproturon (0) > chlorotoluron (1 Cl) > diuron (2 Cl) > fluometuron (3 F)), and the degradation efficiencies of oxidants from fastest to slowest were: O3, ClO2, and Cl2. NDMA can be generated directly from the ozonation of PUHs. Further, compared with chloramination alone, ozonation prominently promoted NDMA formation potential (NDMA-FP) during post-chloramination, and NDMA-FPs increased approximately 23–68 times than those during ozonation only at 2.5 mg/L O3 over 10 min; molar yields of NDMA from highest to lowest were 11.1% (isoproturon), 1.17% (chlorotoluron), 1.0% (diuron), and 0.73% (fluometuron). The PUH degradation kinetics data during ozonation agreed with the pseudo-first-order model. The rate constant kobs were 0.31 × 10-3–19.8 × 10-3 s−1. The kobs and removal ratios of PUHs during ozonation partially scaled with the mass, LogKow, and Henry′s constants of PUHs. Comparisons of measured NDMA-FPs with calculated NDMA-FPs from residual PUH after oxidation showed that the intermediates produced during ozonation facilitated NDMA-FPs; this contribution was also observed for chlorotoluron and isoproturon during ClO2 oxidation. Examination of reaction mechanisms revealed that tertiary amine ozonation, N-dealkylation, hydroxylation, the cleavage of N-C bonds, ammonification, and nitrification occurred during the ozonation of PUHs, and the dimethylamine (DMA) functional groups could be decomposed directly and transformed into NDMA via the formation of the intermediate unsymmetrical dimethylhydrazine. NDMA is also formed from the reaction between DMA and phenylamino-compounds. Clarifying primary degradation products of PUHs and transformation pathways of NDMA during oxidation processes is useful to optimize treatment processes for water supplies.
中文翻译:

在各种氧化条件下苯脲除草剂的降解和N-亚硝基二甲胺的形成:关系和转化途径
在三种氧化条件(氯(Cl 2),二氧化氯(ClO 2)和臭氧(O 3))下,评估了四种苯脲除草剂(PUHs)的降解和转化为N-亚硝基二甲基胺(NDMA)。去除率与PUHs中所含卤素元素的数量有关(异丙隆(0) >氯甲苯(1 Cl) >敌草隆(2 Cl) >氟隆(3 F)),氧化剂从最快到最慢的降解效率为:O 3,ClO 2和Cl 2。NDMA可以直接从PUH的臭氧化中生成。此外,与单独的氯化作用相比,臭氧化作用显着促进了氯化后作用期间NDMA的形成潜能(NDMA-FP),而仅在2.5 mg / LO 3下10分钟内,NDMA-FP的作用比臭氧作用下增加了23-68倍;从最高到最低的NDMA摩尔收率分别为11.1%(异丙隆),1.17%(氯甲苯隆),1.0%(地隆)和0.73%(氟隆)。臭氧化过程中的PUH降解动力学数据与拟一阶模型一致。速率常数k obs为0.31×10 -3 –19.8×10 -3 s -1。第k OBS臭氧化期间PUHs的和除去率与质量,Logk值部分地缩放ow和PUH的Henry常数。氧化后残留PUH中测得的NDMA-FP与计算出的NDMA-FP的比较表明,臭氧化过程中产生的中间体促进了NDMA-FP。在ClO 2期间也观察到氯甲苯和异丙隆的这种贡献氧化。反应机理的研究表明,在PUH的臭氧化过程中,发生了叔胺臭氧化,N-脱烷基化,羟基化,NC键断裂,氨化和硝化反应,二甲基胺(DMA)官能团可直接分解并通过形成中间体不对称二甲基肼。NDMA也由DMA与苯氨基化合物之间的反应形成。在氧化过程中澄清PUHs的主要降解产物和NDMA的转化途径有助于优化供水的处理过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号