Dyes and Pigments ( IF 4.1 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.dyepig.2020.108991 Paola Sánchez-Portillo , Aime Hernández-Sirio , Carolina Godoy-Alcántar , Pascal G. Lacroix , Vivechana Agarwal , Rosa Santillán , Victor Barba
|
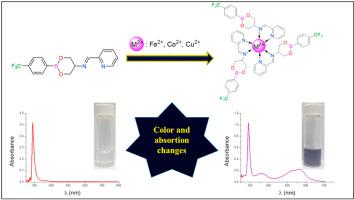
The one-step synthesis of three imine boronic esters functionalized with pyridyl groups is described (1a-1c). The presence or absence of a methyl group affect the whole conformation, being bent “L” or linear as determined by X-ray crystal diffraction. C-H∙∙∙O, C-H∙∙∙N, C-H∙∙∙F and C-H∙∙∙π hydrogen bond interactions support 2D supramolecular arrangements. Ligands were tested as metal ion (M2+) sensors in solution. The addition of metal cations (Fe2+, Co2+ and Cu2+) to 1a using methanol as solvent, showed significant color changes (purple, orange and green, respectively), while for metal cations Ni2+, Zn2+, Cd2+ cations no color changes were observed. The sensitivity of compound 1a towards Fe2+, Co2+ and Cu2+ was monitored by UV-Vis spectroscopy, where the presence of Fe2+ produces new bands at 360 and 566 nm. For Co2+ and Cu2+ a remarkable intensity increase was observed at 290 nm band and new bands appear at 340 and 462 nm, respectively. The stoichiometry of the complexes 1a-Fe2+, 1a-Co2+, 1a-Cu2+ was determined by Job’s plots being 1:3 (metal:ligand, complex [Fe(1a)3]2+), in contrast with the stoichiometry 1:1 observed for 1b-Fe2+. In fact, the computed ΔG formation associated value showed a strong stabilization for the complex [Fe(1a)3]2+ (-65.5 kcal/mol) in comparison with the possible complex [Fe(1b)3]2+ (-29.7 kcal/mol). Detection limits are in the mM range (determined by UV-Vis) and μM range (determined by Fluorescence). An analogous bis-bidentate derivative (2a) also shows similar behavior but stoichiometric interaction changes to 2:3 (metal:ligand) and detection limits are in the mM range obtained by UV-Vis and Fluorescence techniques.
中文翻译:

基于吡啶功能化的亚胺硼酸酯的比色金属离子(II)传感器
描述了用吡啶基官能化的三种亚胺硼酸酯的一步合成(1a - 1c)。甲基的存在或不存在会影响整个构象,如通过X射线晶体衍射所确定的那样弯曲为“ L”或线性。CH∙∙∙O,CH∙∙∙N,CH∙∙∙F和CH∙∙∙π氢键相互作用支持2D超分子排列。测试了配体作为溶液中的金属离子(M 2+)传感器。使用甲醇作为溶剂向1a中添加金属阳离子(Fe 2 +,Co 2+和Cu 2+)显示出明显的颜色变化(分别为紫色,橙色和绿色),而对于金属阳离子Ni 2 +,Zn 2+,Cd 2+阳离子未观察到颜色变化。通过UV-Vis光谱法监测化合物1a对Fe 2 +,Co 2+和Cu 2+的敏感性,其中Fe 2+的存在在360和566 nm处产生新的谱带。对于Co 2+和Cu 2+,在290 nm波段观察到明显的强度增加,并且分别在340和462 nm出现新的波段。复合物的化学计量1A-的Fe 2+,1A -Co 2+,1A-的Cu 2+通过工作的曲线是1确定:3(金属:配体,复合物的[Fe(1a)3 ] 2+),与1b -Fe 2+的化学计量比1:1相反。实际上,与可能的复合物[Fe(1b)3 ] 2 +(-29.7 )相比,计算出的ΔG形成相关值显示出对复合物[Fe(1a)3 ] 2 +(-65.5 kcal / mol)的强稳定性。kcal / mol)。检测限在mM范围(由UV-Vis确定)和μM范围(由荧光确定)中。一个类似的双双导数(2a)也显示出相似的行为,但化学计量相互作用变为2:3(金属:配体),检测极限在通过UV-Vis和荧光技术获得的mM范围内。











































 京公网安备 11010802027423号
京公网安备 11010802027423号