Chemistry and Physics of Lipids ( IF 3.4 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.chemphyslip.2020.105018 C A Marquezin 1 , C M A de Oliveira 2 , F Vandresen 3 , E L Duarte 4 , M T Lamy 4 , C C Vequi-Suplicy 5
|
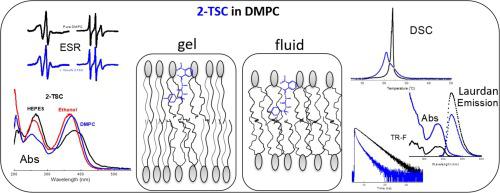
As a potential drug, 2-nitrobenzaldehyde-thiosemicarbazone (2-TSC), a thiosemicarbazone derived from the terpene R-(+)-limonene, was studied through calorimetric and spectroscopic techniques. Differential Scanning Calorimetry (DSC) data showed that 2-TSC causes structural changes in a 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DMPC) membrane, strongly decreasing the cooperativity of the bilayer gel-fluid thermal transition. Optical absorption spectroscopy showed that 2-TSC is more soluble in ethanol and lipids than in water medium, and that the drug displays different structures in the different environments. Though 2-TSC displays no fluorescence, time resolved fluorescence showed that the drug is an effective quencher of the fluorescent probe 6-dodecanoyl-2-dimethylaminonaphthalene (Laurdan). As it is well accepted that Laurdan is positioned into the bilayer close to the membrane surface, that is possibly the localization of 2-TSC in a bilayer. Electron spin resonance (ESR) of the probe 1-palmitoyl-2-stearoyl-(14-doxyl)-sn-glycero-3-phosphocholine (14-PCSL) revealed that 2-TSC is inserted into the hydrocarbon part of the bilayer, fluidizing the lipid bilayer gel phase and rigidifying or organizing the bilayer fluid phase. Similar effects are found for other lipophilic molecules, including cholesterol. These results are useful to improve the understanding of the processes that govern the interaction of thiosemicarbazones with cell membranes, related to the activity of the drugs and their cytotoxicity.
中文翻译:

源自 R - (+) - 柠檬烯的缩氨基硫脲与脂质膜的相互作用
作为一种潜在的药物,2-硝基苯甲醛-缩氨基硫脲(2-TSC),一种衍生自萜烯 R-(+)-柠檬烯的缩氨基硫脲,通过量热和光谱技术进行了研究。差示扫描量热法 (DSC) 数据显示 2-TSC 导致1,2-二棕榈酰-sn-甘油-3-磷酸胆碱(DMPC) 膜的结构变化,强烈降低双层凝胶-流体热转变的协同性。光吸收光谱表明2-TSC在乙醇和脂质中的溶解度高于在水介质中的溶解度,并且药物在不同的环境中表现出不同的结构。尽管 2-TSC 不显示荧光,但时间分辨荧光表明该药物是荧光探针的有效猝灭剂6-十二烷酰基-2-二甲氨基萘(Laurdan)。众所周知,Laurdan 位于靠近膜表面的双层中,这可能是 2-TSC 在双层中的定位。探针1-palmitoyl-2-stearoyl-(14-doxyl)-sn-glycero-3-phosphocholine (14-PCSL) 的电子自旋共振 (ESR)显示 2-TSC 插入到双层的碳氢化合物部分,使脂质双层凝胶相流化并使双层流体相硬化或组织化。对于其他亲脂性分子,包括胆固醇,也发现了类似的效果。这些结果有助于提高对控制缩氨基硫脲与细胞膜相互作用的过程的理解,这些过程与药物的活性及其细胞毒性有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号