Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-11-21 , DOI: 10.1016/j.apsb.2020.11.011 Maria M Disotuar 1 , Jake A Smith 1 , Jinze Li 1 , Steve Alam 1 , Nai-Pin Lin 1, 2 , Danny Hung-Chieh Chou 1, 2
|
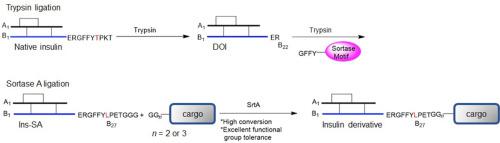
Insulin derivatives such as insulin detemir and insulin degludec are U.S. Food and Drug Administration (FDA)-approved long-acting insulin currently used by millions of people with diabetes. These derivatives are modified in C-terminal B29 lysine to retain insulin bioactivity. New and efficient methods for facile synthesis of insulin derivatives may lead to new discovery of therapeutic insulin. Herein, we report a new method using sortase A (SrtA)-mediated ligation for the synthesis of insulin derivatives with high efficiency and functional group tolerance in the C-terminal B chain. This new insulin molecule (Ins-SA) with an SrtA-recognizing motif can be conjugated to diverse groups with N-terminal oligoglycines to generate new insulin derivatives. We further demonstrated that a new insulin derivative synthesized by this SrtA-mediated ligation shows strong cellular and in vivo bioactivity. This enzymatic method can therefore be used for future insulin design and development.
中文翻译:

通过分选酶 A 连接轻松合成胰岛素融合衍生物
地特胰岛素和德谷胰岛素等胰岛素衍生物是美国食品和药物管理局 (FDA) 批准的长效胰岛素,目前被数百万糖尿病患者使用。这些衍生物在 C 末端 B29 赖氨酸中进行了修饰,以保留胰岛素生物活性。易于合成胰岛素衍生物的新的有效方法可能会导致治疗性胰岛素的新发现。在此,我们报告了一种使用分选酶 A (SrtA) 介导的连接合成胰岛素衍生物的新方法,该方法在 C 端 B 链中具有高效和官能团耐受性。这种具有 SrtA 识别基序的新胰岛素分子 (Ins-SA) 可以与具有 N 端寡甘氨酸的不同基团缀合以产生新的胰岛素衍生物。体内生物活性。因此,这种酶促方法可用于未来的胰岛素设计和开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号