当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and dynamics of an aqueous solution containing poly-(acrylic acid) and non-ionic surfactant octaethylene glycol n-decyl ether (C10E8) aggregates and their complexes investigated by molecular dynamics simulations
Soft Matter ( IF 2.9 ) Pub Date : 2020-11-20 , DOI: 10.1039/d0sm01322f Lakshmikumar Kunche 1, 2, 3, 4, 5 , Upendra Natarajan 1, 2, 3, 4, 5
Soft Matter ( IF 2.9 ) Pub Date : 2020-11-20 , DOI: 10.1039/d0sm01322f Lakshmikumar Kunche 1, 2, 3, 4, 5 , Upendra Natarajan 1, 2, 3, 4, 5
Affiliation
|
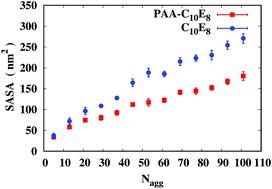
A detailed molecular dynamics simulation study of the self-assembly, intermolecular structure and thermodynamic behavior of an aqueous solution of non-ionic surfactant octa ethylene glycol n-decyl ether (C10E8) in the presence of a non-ionic polar polymer poly(acrylic acid) PAA is presented. The aggregation number Nagg and concentration of surfactant Cs in the simulation systems were varied in the range 0.01–0.32 M and 5 < Nagg < 101 (dilute to concentrated) with a dilute polymer concentration (Cp = 0.01 M). Lamellar aggregates of non-ionic surfactant in bulk aqueous solution are shown by molecular level computations for the first time. Spherical micellar aggregates and lamellar aggregates are formed at low and high Nagg, respectively. The transition from the spherical micelle phase to the lamellar phase in a binary solution is captured for the first time. A conformational transition from coiled to extended PAA chains adsorbed on the surfactant aggregate occurs at a particular value of Nagg, commensurate with the transition from spherical micelle aggregates to anisotropic lamellar aggregates. Formation of the surfactant aggregate in binary and ternary solutions and the polymer–surfactant complex in a ternary solution is enthalpically favored. Adsorption of PAA on the surfactant aggregate surface is driven by hydrogen bonds (HBs) between carboxylic acid groups of PAA and ethylene oxide groups of C10E8. A significant number of HBs occur between polar oxygens of C10E8 and hydroxyl oxygens of PAA. The results are in agreement with the limited available experimental data on this system.
中文翻译:

通过分子动力学模拟研究了含有聚丙烯酸和非离子表面活性剂八乙二醇正癸醚(C10E8)团聚体及其配合物的水溶液的结构和动力学
在非离子极性聚合物聚存在下,非离子表面活性剂八醇乙二醇正癸醚(C 10 E 8)水溶液的自组装,分子间结构和热力学行为的详细分子动力学模拟研究提出了(丙烯酸)PAA。在模拟系统中,聚集数N agg和表面活性剂C s的浓度在0.01-0.32 M范围内变化,并且5 < N agg <101(稀释至浓缩),聚合物浓度稀(C p= 0.01 M)。首次通过分子水平计算显示了本体水溶液中非离子表面活性剂的层状聚集体。球形胶束聚集体和层状聚集体分别在低和高N agg下形成。首次捕获了二元溶液中从球形胶束相到层状相的跃迁。吸附在表面活性剂聚集体上的从卷曲PAA链延伸到延伸PAA链的构象转变发生在特定的N agg与球形胶束聚集体向各向异性层状聚集体的转变相称。在二元和三元溶液中形成表面活性剂聚集体,在三元溶液中形成聚合物-表面活性剂络合物在焓上是有利的。PAA在表面活性剂聚集体表面上的吸附是由PAA的羧酸基团与C 10 E 8的环氧乙烷基团之间的氢键(HBs)驱动的。在C 10 E 8的极性氧与PAA的羟基氧之间存在大量的HBs 。结果与该系统上有限的可用实验数据一致。
更新日期:2020-11-21
中文翻译:

通过分子动力学模拟研究了含有聚丙烯酸和非离子表面活性剂八乙二醇正癸醚(C10E8)团聚体及其配合物的水溶液的结构和动力学
在非离子极性聚合物聚存在下,非离子表面活性剂八醇乙二醇正癸醚(C 10 E 8)水溶液的自组装,分子间结构和热力学行为的详细分子动力学模拟研究提出了(丙烯酸)PAA。在模拟系统中,聚集数N agg和表面活性剂C s的浓度在0.01-0.32 M范围内变化,并且5 < N agg <101(稀释至浓缩),聚合物浓度稀(C p= 0.01 M)。首次通过分子水平计算显示了本体水溶液中非离子表面活性剂的层状聚集体。球形胶束聚集体和层状聚集体分别在低和高N agg下形成。首次捕获了二元溶液中从球形胶束相到层状相的跃迁。吸附在表面活性剂聚集体上的从卷曲PAA链延伸到延伸PAA链的构象转变发生在特定的N agg与球形胶束聚集体向各向异性层状聚集体的转变相称。在二元和三元溶液中形成表面活性剂聚集体,在三元溶液中形成聚合物-表面活性剂络合物在焓上是有利的。PAA在表面活性剂聚集体表面上的吸附是由PAA的羧酸基团与C 10 E 8的环氧乙烷基团之间的氢键(HBs)驱动的。在C 10 E 8的极性氧与PAA的羟基氧之间存在大量的HBs 。结果与该系统上有限的可用实验数据一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号