当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton transfer vs. oligophosphine formation by P–C/P–H σ-bond metathesis: decoding the competing Brønsted and Lewis type reactivities of imidazolio-phosphines
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-11-09 , DOI: 10.1039/d0dt03633a Mario Cicač-Hudi 1, 2, 3, 4 , Christoph M. Feil 1, 2, 3, 4 , Nicholas Birchall 1, 2, 3, 4 , Martin Nieger 5, 6, 7, 8 , Dietrich Gudat 1, 2, 3, 4
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-11-09 , DOI: 10.1039/d0dt03633a Mario Cicač-Hudi 1, 2, 3, 4 , Christoph M. Feil 1, 2, 3, 4 , Nicholas Birchall 1, 2, 3, 4 , Martin Nieger 5, 6, 7, 8 , Dietrich Gudat 1, 2, 3, 4
Affiliation
|
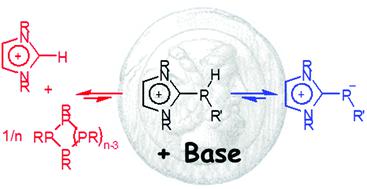
Studies of the protonation and alkylation of imidazolio-phosphides and deprotonation of imidazolio-phosphines reveal a complex behaviour that can be traced back to an interplay of Brønsted-type proton transfers and Lewis-type P–P bond formation reactions. As a consequence, the expected (de)protonation and (de)alkylation processes compete with reactions producing cyclic or linear oligophosphines. A careful adjustment of the conditions allows us to selectively address each reaction channel and devise specific synthesis methods for primary, secondary and tertiary imidazolio-phosphines, imidazolio-alkylphosphides, and cyclic oligophosphines, respectively. Mechanistic studies reveal that oligophosphines assemble in sequential P–P bond formation steps involving the condensation of cationic imidazolio-phosphines via σ-bond metathesis and concomitant elimination of an imidazolium ion. Imidazolio-phosphides catalyse these transformations. Computational model studies suggest that the metathesis proceeds in two stages via an initial nucleophilic substitution under expulsion of a carbene, and a subsequent proton transfer step that generates an imidazolium cation and provides the driving force for the whole transformation. As energy barriers are predicted to be low or even absent, different elementary steps are presumed to form a network of mutually coupled equilibrium processes. Cyclic oligophosphines or their dismutation products are identified as the thermodynamically favoured final products in the reaction network.
中文翻译:

通过P–C / P–Hσ键复分解形成的质子转移与低聚膦:解码咪唑啉膦的竞争性Brønsted和Lewis型反应性
对咪唑并磷化物的质子化和烷基化以及咪唑并膦的去质子化的研究表明,其复杂的行为可以追溯到布朗斯台德型质子转移和路易斯型P–P键形成反应之间的相互作用。结果,预期的(脱)质子化和(脱)烷基化过程与产生环状或线性低膦的反应竞争。仔细调节条件可使我们选择性地处理每个反应通道,并分别设计分别用于伯,仲和叔咪唑啉膦,咪唑烷基磷化物和环状寡膦的特定合成方法。机理研究表明,低聚膦酸酯在依次的P-P键形成步骤中组装,涉及阳离子咪唑并膦通过σ键易位并伴随消除咪唑离子。咪唑磷化物催化这些转化。计算模型研究表明,复分解反应通过卡宾驱逐下的亲核取代反应和随后的质子转移步骤(包括生成咪唑鎓阳离子并提供整个转化的驱动力)而分两个阶段进行。由于预测的能垒很低甚至不存在,因此推测基本步骤不同,以形成相互耦合的平衡过程的网络。环状低膦或其歧化产物被确定为反应网络中热力学上有利的终产物。
更新日期:2020-11-21
中文翻译:

通过P–C / P–Hσ键复分解形成的质子转移与低聚膦:解码咪唑啉膦的竞争性Brønsted和Lewis型反应性
对咪唑并磷化物的质子化和烷基化以及咪唑并膦的去质子化的研究表明,其复杂的行为可以追溯到布朗斯台德型质子转移和路易斯型P–P键形成反应之间的相互作用。结果,预期的(脱)质子化和(脱)烷基化过程与产生环状或线性低膦的反应竞争。仔细调节条件可使我们选择性地处理每个反应通道,并分别设计分别用于伯,仲和叔咪唑啉膦,咪唑烷基磷化物和环状寡膦的特定合成方法。机理研究表明,低聚膦酸酯在依次的P-P键形成步骤中组装,涉及阳离子咪唑并膦通过σ键易位并伴随消除咪唑离子。咪唑磷化物催化这些转化。计算模型研究表明,复分解反应通过卡宾驱逐下的亲核取代反应和随后的质子转移步骤(包括生成咪唑鎓阳离子并提供整个转化的驱动力)而分两个阶段进行。由于预测的能垒很低甚至不存在,因此推测基本步骤不同,以形成相互耦合的平衡过程的网络。环状低膦或其歧化产物被确定为反应网络中热力学上有利的终产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号