当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can molecular flexibility control crystallization? The case of para substituted benzoic acids
Chemical Science ( IF 7.6 ) Pub Date : 2020-11-16 , DOI: 10.1039/d0sc05424k Sin Kim Tang 1 , Roger J Davey 1 , Pietro Sacchi 1 , Aurora J Cruz-Cabeza 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-11-16 , DOI: 10.1039/d0sc05424k Sin Kim Tang 1 , Roger J Davey 1 , Pietro Sacchi 1 , Aurora J Cruz-Cabeza 1
Affiliation
|
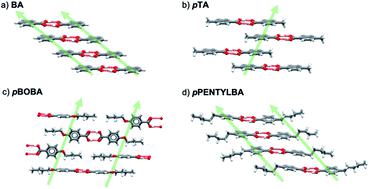
Despite the technological importance of crystallization from solutions almost nothing is known about the relationship between the kinetic process of nucleation and the molecular and crystal structures of a crystallizing solute. Nowhere is this more apparent than in our attempts to understand the behavior of increasingly large, flexible molecules developed as active components in the pharmaceutical arena. In our current contribution we develop a general protocol involving a combination of computation (conformation analysis, lattice energy), and experiment (measurement of nucleation rates), and show how significant advances can be made. We present the first systematic study aimed at quantifying the impact of molecular flexibility on nucleation kinetics. The nucleation rates of 4 para substituted benzoic acids are compared, two of which have substituents with flexible chains. In making this comparison, the importance of normalizing data to account for differing solubilities is highlighted. These data have allowed us to go beyond popular qualitative descriptors such ‘crystallizability’ or ‘crystallization propensity’ in favour of more precise nucleation rate data. Overall, this leads to definite conclusions as to the relative importance of solution chemistry, solid-state interactions and conformational flexibility in the crystallization of these molecules and confirms the key role of intermolecular stacking interactions in determining relative nucleation rates. In a more general sense, conclusions are drawn as to conditions under which conformational change may become rate determining during a crystallization process.
中文翻译:

分子柔性可以控制结晶吗?对位取代苯甲酸的情况
尽管从溶液中结晶具有技术重要性,但对于成核动力学过程与结晶溶质的分子和晶体结构之间的关系几乎一无所知。这一点在我们试图了解制药领域中作为活性成分开发的越来越大、更灵活的分子的行为中表现得最为明显。在我们目前的贡献中,我们开发了一个包含计算(构象分析、晶格能量)和实验(成核率测量)组合的通用协议,并展示了如何取得重大进展。我们提出了第一项旨在量化分子柔韧性对成核动力学影响的系统研究。4对的成核率比较了取代的苯甲酸,其中两种具有带柔性链的取代基。在进行这种比较时,强调了归一化数据以解释不同溶解度的重要性。这些数据使我们能够超越流行的定性描述符,例如“结晶性”或“结晶倾向”,以支持更精确的成核率数据。总体而言,这得出了关于溶液化学、固态相互作用和构象灵活性在这些分子结晶中的相对重要性的明确结论,并证实了分子间堆积相互作用在确定相对成核率中的关键作用。在更一般的意义上,得出的结论是在结晶过程中构象变化可能成为速率决定因素的条件。
更新日期:2020-11-21
中文翻译:

分子柔性可以控制结晶吗?对位取代苯甲酸的情况
尽管从溶液中结晶具有技术重要性,但对于成核动力学过程与结晶溶质的分子和晶体结构之间的关系几乎一无所知。这一点在我们试图了解制药领域中作为活性成分开发的越来越大、更灵活的分子的行为中表现得最为明显。在我们目前的贡献中,我们开发了一个包含计算(构象分析、晶格能量)和实验(成核率测量)组合的通用协议,并展示了如何取得重大进展。我们提出了第一项旨在量化分子柔韧性对成核动力学影响的系统研究。4对的成核率比较了取代的苯甲酸,其中两种具有带柔性链的取代基。在进行这种比较时,强调了归一化数据以解释不同溶解度的重要性。这些数据使我们能够超越流行的定性描述符,例如“结晶性”或“结晶倾向”,以支持更精确的成核率数据。总体而言,这得出了关于溶液化学、固态相互作用和构象灵活性在这些分子结晶中的相对重要性的明确结论,并证实了分子间堆积相互作用在确定相对成核率中的关键作用。在更一般的意义上,得出的结论是在结晶过程中构象变化可能成为速率决定因素的条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号