当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
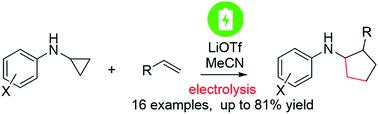
Electrocatalytic redox neutral [3 + 2] annulation of N-cyclopropylanilines and alkenes
Chemical Science ( IF 8.4 ) Pub Date : 2020-11-09 , DOI: 10.1039/d0sc05665k Qi Wang 1 , Qile Wang 2 , Yuexiang Zhang 3 , Yasmine M Mohamed 1 , Carlos Pacheco 1 , Nan Zheng 2 , Richard N Zare 4 , Hao Chen 1
Chemical Science ( IF 8.4 ) Pub Date : 2020-11-09 , DOI: 10.1039/d0sc05665k Qi Wang 1 , Qile Wang 2 , Yuexiang Zhang 3 , Yasmine M Mohamed 1 , Carlos Pacheco 1 , Nan Zheng 2 , Richard N Zare 4 , Hao Chen 1
Affiliation
|
Although synthetic organic electrochemistry (EC) has advanced significantly, net redox neutral electrosynthesis is quite rare. Two approaches have been employed to achieve this type of electrosynthesis. One relies on turnover of the product by the reactant in a chain mechanism. The other involves both oxidation on the anode and reduction on the cathode in which the radical cation or the radical anion of the product has to migrate between two electrodes. Herein, a home-built electrochemistry/mass spectrometry (EC/MS) platform was used to generate an N-cyclopropylaniline radical cation electrochemically and to monitor its reactivity toward alkenes by mass spectrometry (MS), which led to the discovery of a new redox neutral reaction of intermolecular [3 + 2] annulation of N-cyclopropylanilines and alkenes to provide an aniline-substituted 5-membered carbocycle via direct electrolysis (yield up to 81%). A chain mechanism, involving the regeneration of the substrate radical cation and the formation of the neutral product, is shown to be responsible for promoting such a redox neutral annulation reaction, as supported by experimental evidence of EC/MS.
中文翻译:

N-环丙基苯胺和烯烃的电催化氧化还原中性 [3 + 2] 环化
尽管合成有机电化学(EC)取得了长足的进步,但净氧化还原中性电合成却相当罕见。已经采用两种方法来实现这种类型的电合成。一种依赖于链式机制中反应物对产物的周转。另一种涉及阳极上的氧化和阴极上的还原,其中产物的自由基阳离子或自由基阴离子必须在两个电极之间迁移。在此,使用自制的电化学/质谱 (EC/MS) 平台以电化学方式生成N-环丙基苯胺自由基阳离子,并通过质谱 (MS) 监测其对烯烃的反应性,从而发现了一种新的氧化还原N的分子间[3 + 2]环化的中性反应-环丙基苯胺和烯烃通过直接电解提供苯胺取代的 5 元碳环(产率高达 81%)。正如 EC/MS 的实验证据所支持的,涉及底物自由基阳离子的再生和中性产物形成的链式机制被证明是促进这种氧化还原中性环化反应的原因。
更新日期:2020-11-21
中文翻译:

N-环丙基苯胺和烯烃的电催化氧化还原中性 [3 + 2] 环化
尽管合成有机电化学(EC)取得了长足的进步,但净氧化还原中性电合成却相当罕见。已经采用两种方法来实现这种类型的电合成。一种依赖于链式机制中反应物对产物的周转。另一种涉及阳极上的氧化和阴极上的还原,其中产物的自由基阳离子或自由基阴离子必须在两个电极之间迁移。在此,使用自制的电化学/质谱 (EC/MS) 平台以电化学方式生成N-环丙基苯胺自由基阳离子,并通过质谱 (MS) 监测其对烯烃的反应性,从而发现了一种新的氧化还原N的分子间[3 + 2]环化的中性反应-环丙基苯胺和烯烃通过直接电解提供苯胺取代的 5 元碳环(产率高达 81%)。正如 EC/MS 的实验证据所支持的,涉及底物自由基阳离子的再生和中性产物形成的链式机制被证明是促进这种氧化还原中性环化反应的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号